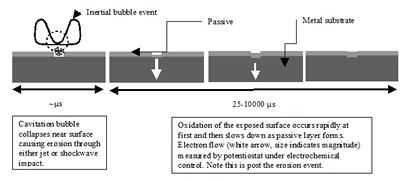
Research project: Birkin: Surface Erosion/Corrosion
The effect of power ultrasound on surfaces has been well documented. Indeed the presence of cavitation generated by flow, sound or some other technique is well known to have a detrimental effect on a surface. However, this characteristic can be used to quantify the presence of erosive mechanisms in this environment.