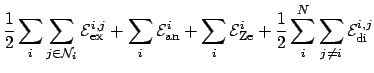 |
(2.15) |
Combining equations 2.4, 2.9, 2.11 and 2.14 yields the total energy:
The number of atoms in comparatively small systems is large. Assuming
a cubic structure and a lattice spacing of 2.5Å as in iron, cobalt or
nickel, a cube of edge length 100nm would contain 6.4![]() 10
10![]() atoms.
atoms.