Background and Aims of the Trial
Chronic kidney disease is very common, 13% of UK adults have CKD (any stage) and 6-7% of UK adults have CKD G3-5 (stages 3-5).
Only a minority of people with CKD will ever need kidney dialysis or transplantation, but all are at increased risk of cardiovascular disease.
In patients who already have cardiovascular disease, antiplatelets (predominantly aspirin) have been shown to reduce the risk of further attacks and that these benefits are much greater than the risks of bleeding.
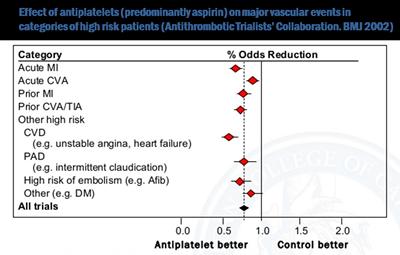
As a result, aspirin is internationally recommended for people who have already had a heart attack or stroke.
Aspirin is less beneficial in preventing a first attack or stroke in the general low risk population and is not recommended for this purpose.
Heart attacks and strokes however, are far more common in people with CKD than in the general population, so we would expect aspirin to be of greater benefit. On the other hand, the risks may also be higher as bleeding is more common in people with reduced kidney function.
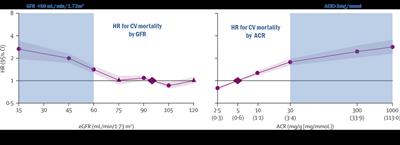
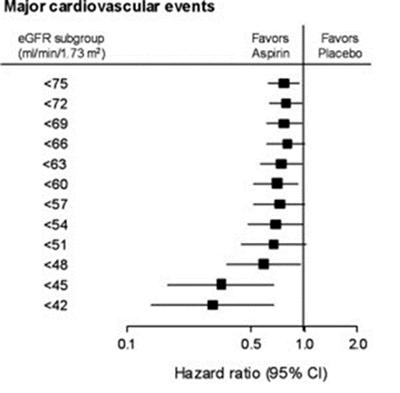
Before we can recommend aspirin treatment to help a first heart attack or stroke in people with CKD, we need to be sure that the benefits of treatment outweigh the possible risks. There is some evidence to suggest that the relative benefits of aspirin in preventing CVD are greater in those with reduced kidney function.
The National Institute of Health and Care Excellence (NICE) therefore recommended that a definitive research study was needed (2014 NICE CKD Guideline).
Trial Design
ATTACK is a pragmatic multicentre open-label randomised controlled trial.
Participants will be aged 18+ with CKD (Stage 1-4), with no pre-existing cardiovascular disease or a pre-existing condition associated with increased risk of bleeding, and not taking aspirin or anticoagulants.
Eligible participants will be randomised (1:1) to take low-dose aspirin (75mg) once daily in addition to regular prescribed medication, OR continue with regular medication alone.
The trial will be managed from three Regional Centres based in Southampton, Midlands (Nottingham and Warwick) and Newcastle-upon-Tyne.
The workload for GP Practices is small. The ATTACK toolkit will search for eligible patients, GPs perform a concise check of the search list and invitation letters are sent out by Docmail (automatically to eligible patients via the ATTACK toolkit). All trial follow-up for CVD and bleeding outcomes will be done routinely by linkage to GP, hospitalisation (HES) and mortality (ONS) data.
Funder
Funded jointly by:
National Institute for Health Research Health Technology Assessment Programme (HTA Project: 16/31/127)
and the British Heart Foundation (SP/17/14/33355)