Current research projects:
Photocatalytic fibres for sustainable applications
Optical fibres offer a unique way to introduce light into photochemical reactors. By coating the interior of hollow optical fibres with photocatalysts I create all-in-one photocatalytic reactors. In the fibres interior the photocatalyst, reagents and photons can effectively mix. We are expanding the range of photocatalytic materials we can incorporate into these fibres and the reactions we can do with them.
M. E. Potter et al, Combining Photocatalysis and Optical Fiber Technology toward Improved Microreactor Design for Hydrogen Generation with Metallic Nanoparticles, ACS Photonics, 2020, 7, 714.
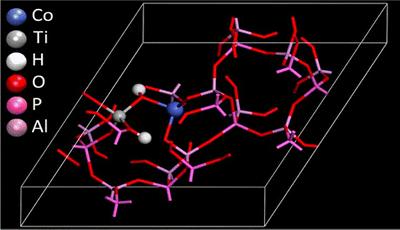
Combining calculations and characterisation to design multi-metallic catalysts
Density Functional Theory calculations can provide a wealth of information at the electronic level for isolated active sites in framework materials. Combining this with experimental data from synchrotron source, specifically X-ray adsorption spectroscopy (Diamond, RAL), means it is possible to verify these models. This allows us to develop new synthesis methods to control the proximity of complimentary active sites towards catalytic synergy.
M. E. Potter et al, Spectroscopic and Computational Insights on Catalytic Synergy in Bimetallic Aluminophosphate Catalysts, J. Am. Chem. Soc., 2015, 137, 8534.
M. E. Potter et al, Theoretical insights into the nature of synergistic enhancement in bimetallic CoTiAlPO-5 catalysts for ammonia activation, Catal. Sci. Technol., 2017, 7, 3474.
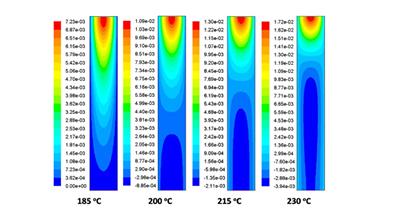
Improving reactor design through computational fluid dynamics modelling
Catalytic data is a vital input for developing working kinetic models. By careful analysis of the product distribution it is possible to unravel the detailed kinetics, and catalytic pathways, that occur within a process. Combining this with computational fluid dynamics allows one to identify important real-life features such as temperature hot-spots and required flow rates towards optimal process design.
M. E. Potter et al, Combining catalysis and computational fluid dynamics towards improved process design for ethanol dehydration, Catal Sci. Technol. 2018, Accepted.
M. E. Potter et al, Role of Isolated Acid Sites and Influence of Pore Diameter in the Low-Temperature Dehydration of Ethanol, ACS Catal., 2014, 4, 4161.
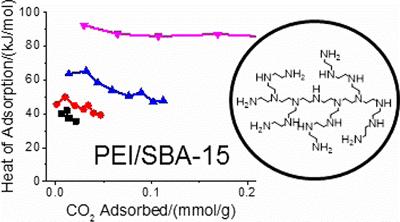
Designing amine-based materials for carbon capture
Atmospheric CO2 is a major issue in modern society that demands our attention. Solid sorbents, such as aminosilane species are able to adsorb significant amounts of CO2 if designed correctly. The binding strength of CO2 is a crucial metric in direct air capture, which is influenced by many factors, most notably support-amine interactions. By understanding these factors we hope to optimise these species for industrial CO2 capture.
M. E. Potter et al, Role of Alumina Basicity in CO2 Uptake in 3-Aminopropylsilyl-Grafted Alumina Adsorbents, ChemSusChem, 2017, 10, 2192.
M. E. Potter et al, Adsorption Microcalorimetry of CO2 in Confined Aminopolymers, Langmuir, 2017, 33, 117.
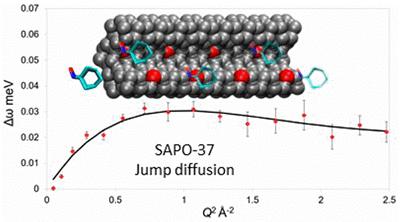
Understanding catalytic processes with neutron scattering techniques
Porous materials are of great interest for industrial catalytic applications, as they can control the size of the reagents and products, therefore steering reactions towards specific pathways. Therefore diffusion must be carefully monitored and controlled towards optimising porosity. In collaboration with ISIS (RAL) we probe the diffusion and interactions between porous materials and reactants with neutron scattering techniques to explore reaction pathways.
M. E. Potter et al, Spectroscopic investigation into the design of solid–acid catalysts for the low temperature dehydration of ethanol, Phys. Chem. Chem. Phys., 2016, 18, 17303.
M. E. Potter et al, Understanding the Role of Molecular Diffusion and Catalytic Selectivity in Liquid-Phase Beckmann Rearrangement, ACS Catal., 2017, 7, 2926.