Imprisoned molecules ‘quantum rattle’ in their cages
Scientists have discovered that a space inside a special type of carbon molecule can be used to imprison other smaller molecules, such as hydrogen or water.
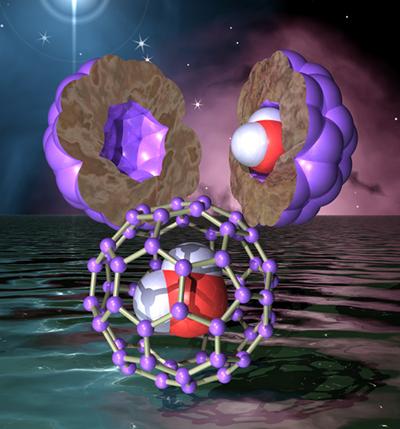
The nano-metre sized cavity of the hollow spherical C60 Buckminsterfullerene — or ‘bucky ball’ — effectively creates a ‘nanolaboratory’, allowing detailed study of the quantum mechanical principles that determine the motion of the caged molecule, including the mysterious wave-like behaviour that is a fundamental property of all matter.
Experiments by the international collaboration of researchers, including chemists from the University of Southampton, have revealed the wave-like behaviour and show how the imprisoned H2 and H2O molecules ‘quantum rattle’ in their cage.
Professor Malcolm Levitt, Head of Magnetic Resonance at the University of Southampton, who has used the technique nuclear magnetic resonance (NMR) to study the quantum properties of the caged molecules, said: “By confining small molecules such as water in fullerene cages we provide the controlled environment of a laboratory, but on the scale of about one nanometre.
“Under these conditions, the confined molecules reveal a wave-like nature and behave according to the laws of quantum mechanics. Apart from their intrinsic interest, we expect that the special properties of these materials will lead to a variety of applications, such as new ways to brighten the images of MRI scans, and new types of computer memory.”
The research, which involved scientists from the US, Japan, France, Estonia and the universities of Nottingham and Southampton in the UK, has recently been published in the prestigious journal Proceedings of the National Academy of Sciences (PNAS).
The discovery of the C60 Buckminsterfullerene, and the related class of molecules the fullerenes, in the mid-1980s earned Professors Harry Kroto, Robert Curl and the late Richard Smalley the Nobel Prize in Chemistry in 1996.
It has a cage-like spherical structure made up for 20 hexagons and 12 pentagons and resembles a soccer ball, earning it the nickname ‘bucky ball’.
In a recent breakthrough in synthetic chemistry, the Japanese scientists from Kyoto have invented a molecular surgery technique allowing them to successfully permanently seal small molecules such as H2 and H2O inside C60.
They used a set of surgical synthetic procedures to open the C60 ‘cage’ producing an opening large enough to ‘push’ a H2 or H2O molecule inside at high temperature and pressure. The system was then cooled down to stabilise the entrapped molecule inside and the cage was surgically repaired to reproduce a C60.
The work published in the PNAS paper has also separately identified two subtly different forms of H2O — ortho-water and para-water. These so called nuclear spin-isomers also owe their separate identities to quantum mechanical principles.
What's related
We expect that the special properties of these materials will lead to a variety of applications, such as new ways to brighten the images of MRI scans, and new types of computer memory.