Novel catalyst design signals key step for sustainable manufacture of nylon
Researchers at the University of Southampton have developed a versatile and durable solid catalyst that can be used to produce synthetic polymers more sustainably.
A multidisciplinary international collaboration, led by Dr Stephanie Chapman and Professor Robert Raja, was instrumental in the design of the novel hierarchical catalyst, which produces a nylon precursor in high yield.
The industrial catalyst could be used in the sustainable manufacture of Nylon-6, or polycaprolactam, a polymer that is used in a range of materials including carpets, seat belts and parachutes.
Scientists have published their findings online in Angewandte Chemie, a journal of the German Chemical Society, GDCh.
A catalyst is a material that can speed up a chemical reaction. Catalysts are fundamental to ‘Green Chemistry’ because they help to make chemical reactions safer, less wasteful and less energy intensive. These economic and environmental benefits mean that catalysts are used in the majority of industrial processes - from petroleum processing to pharmaceutical manufacture.
Catalysts are also important in the industrial production of synthetic polymers. Nylon-6 is produced through the polymerisation of a cyclic amide that is itself catalysed by a mechanism known as Beckmann rearrangement. The traditional, industrial Beckmann rearrangement is a multi-step process that uses a very strong sulphuric acid catalyst and creates huge quantities of low-value ammonium sulphate by-product.
Solid-acid catalysts of the type designed by the Southampton researchers are being investigated for the Beckmann rearrangement as an alternative to aggressive sulphuric acid, simultaneously eliminating the production of ammonium sulphate, which ends up in landfill. These solid catalysts work under less energy intensive conditions and can be easily recycled, so are ready to be used again.
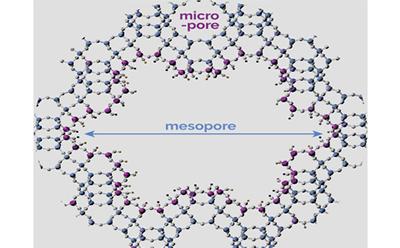
The latest research describes a new hierarchical catalyst structure, which was created using a process called soft-templating. Soft-templating uses a small compound (called a structure-directing agent) along with a larger molecule of the type found in soap (called a surfactant) that act as templates around which the solid catalyst is formed. When the templates are removed, a porous material is formed with interconnected channels on the micro- (< 2 nm) and meso- (2-50 nm) scale.
In a chemical reaction, a porous catalyst acts like a molecular sieve, with targeted chemical transformations occurring inside the pores. A hierarchical catalyst balances the benefits of smaller and larger pores: the smaller pores help to trap the reactant so that it is transformed into the desired product, whilst the larger pores allow compounds to move more easily within the nanoscopic channels, so that they don’t get stuck.
Lead author Dr Stephanie Chapman, who conducted the research within Southampton’s Functional Inorganic, Materials and Supramolecular Chemistry group, says: “Catalytic studies are continuing to improve the sustainability of so many important chemical processes that impact our everyday lives.
“This paper describes how a material design strategy can be used to enhance catalyst performance in an industrially-relevant reaction. With the help of colleagues across the world, we have been able to undertake in-depth characterisation, which has helped us to better understand how the synthetic approach affects the properties of these particular porous catalysts.”
Nylon-6’s strength and resistance to physical and chemical degradation has led to its extensive use in plastics and fibres, with global demand expected to reach 7,690 megatonnes by 2026.
Professor Robert Raja, Professor of Materials Chemistry and Catalysis, who conceived and coordinated this research, says: “Conventional industrial Beckmann rearrangement with microporous catalysts suffers from mass-transport limitations, which reduces catalyst lifetimes; so it is vital that innovative catalyst design solutions are engineered to deliver a sustainable industrial future. I am delighted that this collaborative research effort has been recognised in such a prestigious journal and this excellent outcome reflects the hard work of the whole team.”
The project was part-funded by the European Union through the Horizon 2020 MULTI-site organic-inorganic HYbrid CATalysts for MULTI-step chemical process (MULTI2HYCAT) programme and AdvanSix Inc, a fully integrated manufacturer of Nylon-6 resin.
Co-authors in the Angewandte Chemie article include experts from the STFC Rutherford Appleton Laboratory, Didcot, the Università del Piemonte Orientale, Italy, CSIRO Manufacturing, Australia, and the International Center of Future Science, Jilin University, China.
The latest advances build upon 10 years of collaboration between Professor Raja’s research group at Southampton with industrial partners Honeywell and AdvanSix Inc, which has led to the award of several international patents. Professor Raja will present the findings from this paper at an Angewandte Chemie and Advanced Materials joint symposium on Functional Porous Materials.