Extraordinary molecular discovery provides ‘reality check’ for crystal engineering
An international team of researchers have explained how some of the most complicated crystal structures ever determined are derived from a simple organic salt.
The crystalline mystery, driven by a discovery at the University of Southampton’s National Crystallography Service, has been labelled a “reality check” in the quest to design materials with tunable chemical-physical properties.
Three generations of researchers in Southampton’s School of Chemistry collaborated with experts around the globe to unravel how a simple organic molecule could produce crystalline phases of extraordinary complexity. Their findings have been published in Nature.
Molecular self-assembly, the spontaneous association of single molecules into larger ordered structures, represents a key step in several natural processes, such as the formation of crystals, proteins, viruses or double-helical DNA.
Crystalline materials are formed through the periodic and highly symmetric self-assembly of molecules. These can be designed to contain different physical-chemical properties from this transformation based on their molecular arrangement and intermolecular interactions, triggering opportunities for industrial applications.
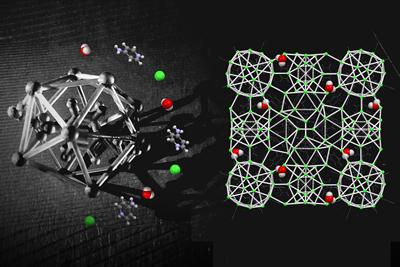
Dr Riccardo Montis first discovered the extremely complex crystalline structure from a chloride salt of the compound fampridine during his postgraduate research at Southampton. The crystal engineer returned to the mystery as a visiting researcher at Southampton for several years and in 2017 isolated a second complex structure based on a sub-hydrate.
“From this moment we realised that the previous structure was not just an unusual and unique case of a complex system, but probably only the tip of the iceberg,” Riccardo says.
Riccardo’s investigation was supported by Professor Micheal Hursthouse, who remains an Emeritus Professor within Chemistry at Southampton, and NCS’s Dr Peter Horton. It included contributions from several members of the Characterisation and Analytics group, particularly Professor Simon Coles and Dr Terry Threlfall.
“These crystal structures represent two of the most complex crystal structures ever isolated for a simple organic molecule,” Simon says. “This discovery represents a reality check in the field of crystal engineering, contributing to the comprehension of the mechanisms of formation of crystals and opening perspectives for the design of new classes of materials with tunable chemical-physical properties.”
The researchers’ latest work has identified that the new crystalline phases might arise from an unusual molecular self-assembly in the liquid state, consisting of spherical aggregates of molecules.
“We are trying to isolate more complex phases of this salt while, at the same time, studying other systems potentially able to generate such complex self-assemblies,” Riccardo says. “We are also investigating the possibility to use the spherical self-assemblies observed in the liquid state as supramolecular building blocks to design new materials.
“This has definitely been a team effort across different generations of researchers. The elder generation provided so much enthusiasm and food for thought, while the younger members contributed with experimental support. There have been endless and fruitful discussions about the system at Southampton and the extension of the mystery around the world helped provide the last and most important pieces of the puzzle.”
Over the years several collaborations have been forged to investigate the solid-state of fampridinium chloride. The team engaged the Australian National University’s Professor Dave Rae, before approaching Professor Gerard Coquerel at the Université de Rouen Normandie and Dr Anais Lafontaine.
In 2016, the study evolved to investigate the self-assembly in solution. The research was advanced with liquid-state NMR and further characterisation in solid-state by Dr Luca Fusaro and Dr Nikolay Tumanov from Belgium’s University of Namur and cryo-EM experiments by Professor Andrea Falqui and Dr Rachid Sougrat at the King Abdullah University of Science and Technology (KAUST) in Saudi Arabia. These collaborations proved to be crucial in identifying the spherical assemblies in the liquid state.