James T. Frith, Luyi Yang, Nuria Garcia-Araez, John R. Owen
Lithium-oxygen cells are seen as the next generation of energy storage devices as they have a theoretical specific energy of around 5 times that of current Li-ion cells. Since being proposed by Abraham and Jiang 1 lithium-oxygen cells have faced a series of setbacks. In particular their development was hindered as a result of reactions between superoxide, a product of oxygen reduction, and commonly used carbonate based organic electrolytes 2,3. The adoption of stable electrolytes has led to great improvements in lithium-oxygen cell chemistry. The greatest problems now facing lithium-oxygen cells are associated with the insulating and insoluble nature of the primary discharge product, lithium peroxide. In particular the cell capacity and cycle life are found to be hindered 4.
This work will first look into how in situ Raman spectroscopy can be used to identify stable electrolytes for use in lithium-oxygen cells 5. The use of homogenous catalysts and how they can be used to avoid problems associated with lithium peroxide deposition on the electrode surface was then examined. The increase in the capacity seen when using ethyl viologen as a catalyst of the oxygen reduction reaction was investigated. This was found to be a direct result of the 2-electron reduction of oxygen as promoted by ethyl viologen 6,7.
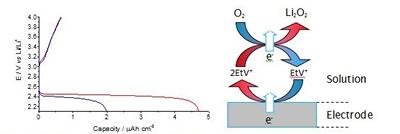
Figure 1. Galvanostatic discharge of a glassy carbon electrode in oxygen saturated 100 mM LiTFSI, Pyr14TFSI with (—) 0 mM EtV(OTf)2, (—) 0.5 mM EtV(OTf)2. Current: 20 µA•cm-2. Diagram - Mode of action of ethyl viologen when catalysing the 2-electron reduction of oxygen in a Li-O2 cell.
1. Abraham, K. M. & Jiang, Z. J. Electrochem. Soc. 143, 1–5 (1996).
2. Mizuno, F., Nakanishi, S., Kotani, Y., Yokoishi, S. & Iba, H. Electrochemistry 78, 403–405 (2010).
3. Freunberger, S. a et al. J. Am. Chem. Soc. 133, 8040–7 (2011).
4. Viswanathan, V. et al. J. Chem. Phys. 135, 214704 (2011).
5. Frith, J. T., Garcia-Araez, N., Russell, A. E. & Owen, J. R. 1–6 (2014).
6. Lacey, M. J., Frith, J. T. & Owen, J. R. 26, 74–76 (2013).
7. Yang, L., Frith, J. T., Garcia-Araez, N. & Owen, J. R. Chem. Commun. 2–5 (2014).