Defining Hydrogen Bonds to Determine the Structure and Dynamics of Water Seminar
- Time:
- 16:00
- Date:
- 11 May 2012
- Venue:
- Building 27, room 2001
For more information regarding this seminar, please email Dr Chris-Kriton Skylaris at C.Skylaris@southampton.ac.uk .
Event details
Despite over a century of research, there is still disagreement about the structure of water as well as its dynamics.
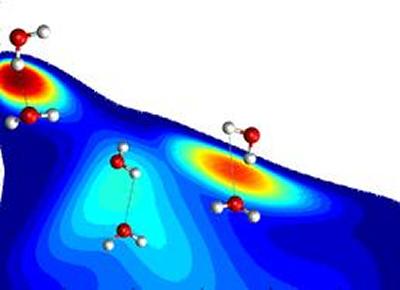
Assuming a maximum of four hydrogen bonds and drawing on evidence of very few broken hydrogen bonds, water is commonly considered to be distorted tetrahedral and hydrogen-bond switching is highly concerted. Yet other data suggests that there is a variable number of hydrogen bonds including broken ones, which suggests a mixture of structures and that switching is stepwise. Much of the dispute hinges on how hydrogen bonds are defined. Most approaches require that a hydrogen bond has a minimum cut-off in strength. However, a single cut-off is either too restrictive or too generous.depending on the arrangement of molecules and fails to locate transition states for donors switching between acceptors. Here we present a simple, parameter-free definition of the hydrogen bond (J Phys Chem B, 2010, 114, 16792) that accurately identifies hydrogen bonds and transition states of hydrogen-bond switching. We present the resulting structure and dynamics of water and show how they resolve the conflicting data.
Speaker information
Dr Richard Henchman, University of Manchester. Lecturer in Theoretical Chemistry