Adventures in Non-Covalent Chemistry: From DNA Bases to alpha-helices Seminar
- Time:
- 16:00
- Date:
- 7 January 2015
- Venue:
- Building 27, Room 2001 Chemistry University of Southampton Southampton SO17 1BJ
For more information regarding this seminar, please email Jon Watts at J.K.Watts@soton.ac.uk .
Event details
Professor Andy Wilson presents a seminar as part of the joint seminar series organised by the Chemical Biology, Diagnostics and Therapeutics Research Group and the Organic Chemistry: Synthesis, Catalysis and Flow Research Group.
Modern challenges in materials and biological science require the ability to understand and manipulate non-covalent chemistry. This presentation will discuss two bio-inspired approaches under development in our group to achieve this objective focused on materials assembly and inhibition of protein-protein interactions.
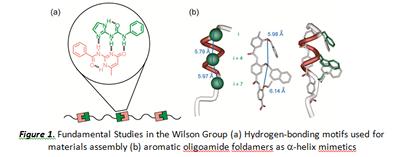
'Smart materials' or stimuli responsive polymers (i.e. those that change their properties in response to temperature, solvent, light or chemical additives) are highly sought after. Their properties like those of biopolymers, are governed by the non-covalent interactions that give rise to defined folded or aggregated architectures. Inspired by the assembly of DNA, the first part of this presentation will focus on our efforts to design hydrogen-bonding motifs[1] and their incorporation into supramolecular polymers (Fig la). [2]
In the case of α-helix mediated PPIs, a number have been shown to represent attractive targets for the development of anticancer therapeutics, however what is not clear is how to do this using a small molecule, given that it must cover 800-1100Å2 of a protein surface and complement the discontinuous projection of hydrophobic and charged domains over a flat or moderately convex surface. The second part of this presentation will focus on our efforts to develop robust synthetic methods for synthesis of Proteomimetics (Fig. 1b) and the associated screening efforts against several targets including p53/hDM2 and Mcl-1/NOXA-B. [3-4]
References
[1] M.L. Pellizzaro, K.A. Houton, A.J. Wilson, Chem. Sci., 2013, 4, 1825-1829
[2] A.Gooch, C.Nedolisa, K. Houton, C.I.Lindsay, A.Saini, A.J.Wilson, Macromolecules, 2012, 4723
[3] V. Azzarito, K.Long, N.S.Murphy and A.J.Wilson, Nature Chem, 2013, 5, 161-173
[4] F. Campbell, J.P.Plante, T.A.Edwards, S.L.Warriner, A,J,Wilson, Org. Biomol. Chem, 2010, 8, 2344-2351
Speaker information
Prof Andrew Wilson, University of Leeds. Professor of Organic Chemistry, School of Chemistry