Novel phosphorus-containing ring systems derived from the 2- phosphaethynolate anion Seminar
- Time:
- 15:00
- Date:
- 22 April 2015
- Venue:
- Building 27, Room 2003 Chemistry University of Southampton Southampton SO17 1BJ
For more information regarding this seminar, please email Geoff Hyett at G.Hyett@soton.ac.uk .
Event details
Jose Goicoechea presents a seminar as part of the FIMS research groups' seminar series
The 2-phosphaethynolate anion (PCO−), first reported by Becker and co-workers,[1]can be readily accessed by carbonylation of the heptaphosphide trianion [P7]3−.[2] This heavier cyanate analogue contains a reactive P−C multiple bond and can be employed as an ambidentate nucleophile or as a precursor to novel phosphorus containing compounds.[3] For example, [2+2] cycloaddition reactions with heteroallenes(carbodiimides and isocyanates) give rise to four-membered rings containing a phosphide vertex.[1] Similarly, PCO− reacts with molecules with unsaturated maingroup element–element bonds also affording novel heteroatomic ring systems.[4]
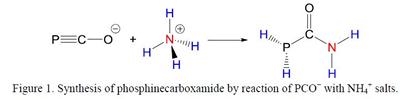
By analogy with Wöhler’s urea synthesis, direct reaction of PCO− with ammonium salts has been shown to yield phosphinecarboxamide (PH2C(O)NH2; Scheme 1), an air- and moisture-stable primary phosphine.[5] This talk will focus on recent developments concerning the chemical reactivity of PCO− and PH2C(O)NH2 for the formation of novel neutral and anionic inorganic ring systems.
Acknowledgements: We thank the University of Oxford and the EPSRC(EP/K039954/1) for financial support.
References:
[1] G. Becker, W. Schwarz, N. Seidler, M. Westerhausen, Z. Anorg. Allg. Chem., 1992, 612, 72.
[2] A. R. Jupp, J. M. Goicoechea, Angew. Chem., Int. Ed., 2013, 52, 10064.
[3] See for example: (a) S. Alidori, D. Heift, G. Santiso-Quinones, Z. Benkő, H. Grützmacher, M. Caporali, L. Gonsalvi, A. Rossin, M. Peruzzini, Chem.−Eur. J., 2012, 18, 14805; (b) X. Chen, S. Alidori, F. F. Puschmann, G. Santiso-Quinones, Z. Benkő, Z. Li, G. Becker, H.-F. Grützmacher, H. Grützmacher, Angew. Chem. Int. Ed., 2014, 53, 1641; (c) D. Heift, Z. Benkő, H. Grützmacher, Angew. Chem. Int. Ed., 2014, 53, 6757; (d) A. M. Tondreau, Z. Benkő, J. R. Harmer, H. Grützmacher, Chem. Sci. 2014, 5, 1545.
[4] T. P. Robinson, M. J. Cowley, D. Scheschkewitz, J. M. Goicoechea, Angew. Chem., Int. Ed., 2015, 54, 683.
[5] A. R. Jupp, J. M. Goicoechea, J. Am. Chem. Soc., 2013, 135, 19131.
Speaker information
Dr Jose M. Goicoechea, University of Oxford. Department of Chemistry