Synthesis and applications of 6-deoxy-6-amino-chitosan Seminar
- Time:
- 14:00
- Date:
- 22 March 2018
- Venue:
- Building 27, Lecture Room 2003, Chemistry, University of Southampton
For more information regarding this seminar, please email Prof Jon Essex at J.W.Essex@soton.ac.uk .
Event details
Seminar with Dr Anwar Jardine
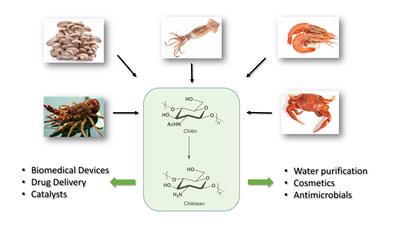
Chitin and chitosan are renewable biopolymers with inherently favourable properties allowing diverse chemical modification that generate novel nanomaterials ideal for catalytic, cosmetic and biomedical applications.1 These polymers are linear semi-crystalline polysaccharides with unique properties including biocompatibility, chemical versatility, biodegradability and low toxicity. Chitin itself has very poor aqueous solubility and hence found limited utility. One of the major challenges in biopolymer valorisation is finding environmentally friendly solvents for chemical modification of the native polymer. Thus, the aqueous-acid soluble chitosan and modified chitosan has increased exponentially, particularly in biomedical applications.
Chitin production can be integrated into the biorefinery concept that exploits the use of fungi, crustaceans and insects fed on food waste. Due to the abundance of chitin waste, it is desirable to find water soluble derivatives for further exploitation. Numerous strategies have been proposed for the conjugation of polar groups to the 6-hydroxy and/or the 2-amino functional groups of chitosan. However, to retain the polymer’s structural skeleton, substitution of the 6-hydroxy for the basic 6-amino group is desirable and imparts water solubility to the chitosan polymer.2 The exploitation of this water soluble 6-deoxy-6-amino chitosan has not been fully realized. Derivatives of 6-deoxy-6-amino chitosan possesses improved antimicrobial activity compared to the native polymer.3,4 However, the manufacturing cost per kg needs to be sufficiently low to appeal to agricultural applications to aid food security. Here we report an improved, short and scalable synthesis of 6-deoxy-6-amino chitin and chitosan.5
1. Jardine, A.; Sayed, S., Challenges in the valorisation of chitinous biomass within the biorefinery concept. Current Opinion in Green and Sustainable Chemistry 2016, 2, 34-39.
2. Satoh, T.; Kano, H.; Nakatani, M.; Sakairi, N.; Shinkai, S.; Nagasaki, T., 6-Amino-6-deoxy-chitosan. Sequential chemical modifications at the C-6 positions of N-phthaloyl-chitosan and evaluation as a gene carrier. Carbohydrate Research 2006, 341 (14), 2406-2413.
3. Goy, R. C.; Assis, O. B. G., Antimicrobial analysis of films processed from chitosan and N,N,N-trimethylchitosan. Brazilian Journal of Chemical Engineering 2014, 31, 643-648.
4. Geng, X.; Yang, R.; Huang, J.; Zhang, X.; Wang, X., Evaluation Antibacterial Activity of Quaternary-Based Chitin/Chitosan Derivatives In Vitro. Journal of Food Science 2013, 78 (1), M90-M97.
5. a) Jardine et al, Polymer Support, US 842 802 B2 b) Jardine et al, Method of Synthesising 6-Deoxy-6-Amino- β-D-Glucopyranoside contianing Polymers, Patent Application No: 1719330.1

Speaker information
Dr Anwar Jardine , University of Cape Town, South Africa. After completion of my PhD in Organic Chemistry at the University of Cape Town (UCT) in 1995, I received an Andrew Mellon Foundation Postdoctoral Research Fellowship Award for studies at UCT Medical School. Here I applied my acquired skills in organic natural product synthesis toward the synthesis of a natural antioxidants. An early highlight of my career was achieving the first chemical synthesis of the mycobacterium tuberculosis antioxidant metabolite called mycothiol. Mycothiol research was given a major boost after synthetic material became accessible. As an extension of the UCT Medical School TB research program, I accepted an invitation from GlaxoSmithkline to spend a year as a postdoctoral scientist at their corporate R&D center in Stevenage (UK). Whilst utilising my natural product chemistry synthetic skills, its application shifted more toward rational drug design. This was a great opportunity to be part of a multi-disciplinary drug discovery group in a major pharmaceutical company. After completing a year on the TB research program, I took up a postdoctoral fellowship at Harvard Medical School. At Harvard Medical School (1998-1999) I conducted natural product chemistry and drug discovery in search of nucleotide based anticancer drugs. In 1999, I left academia and took up a position as Senior Scientist at Johnson Matthey Pharma Outsourcing Division (MA, USA). In the latter position, I engaged in drug development and manufacturing of anti-retrovirals that became the important HIV drugs of today. After spending three years working in the pharmaceutical industry, I was offered a Senior Research Scientist position at Gillette Advanced Technology Centre (MA, US). I joined the Hair Biology and Dermatologics research group where my prior experience in natural products and medicinal chemistry were valued. Being part of a FMCG company, my research was focused on personal care product innovation. While participating in the development of hair growth inhibiting drugs, research in natural product based hair growth inhibitors were actively pursued. Within the latter program I further developed my knowledge and experience in regulatory aspects, interacting with the FDA regarding NDAs (New Drug Applications) as well as patenting, product development and marketing. In 2004, I decided to return to SA to start a career in academia at the University of Stellenbosch where I established a renewed interest in natural product chemistry. However, this time I decided to focus on low molecular mass antioxidants and its role in health and disease. Additionally, I also become passionate about adding value to biomass waste in the context of Green Chemistry. In July 2008, I started a Senior Lectureship at UCT where I continued establishing research capacity in natural product antioxidant biosynthesis and its potential in antimicrobial drug design. In 2009, I was awarded a Grand Challenge Explorations Grant for mycothiol research by the Bill and Melinda Gates Foundation. More recently, I focused on utilising green chemistry principles across all synthesis, whether small molecule natural products or biopolymers. The latter approach led to an expedient synthesis of the lessor known antioxidant vitamin, ergothioneine. Green Chemistry now overarches my entire approach to synthesis, specifically biopolymer valorisation using food waste as a source. The latter research led to the development of water soluble heterogeneous catalysts and antimicrobial polymers. I have also introduced Green Chemistry in my teaching curriculum. I am a member of a Global Green Chemistry Network, G2C2, comprising of members from 36 countries. I also serve as global technical consultant for Unilever.