Synthesis and study of cyanopolyynes and derivatives: from interstellar chemistry to organic synthesis methodology Seminar
- Time:
- 15:00
- Date:
- 25 April 2018
- Venue:
- Building 27, Room 2003 Chemistry University of Southampton SO17 1BJ
Event details
Dr Yann Trolez presents a seminar as part of the Functional Inorganic, Materials and Supramolecular section’s seminar series.
Some cyanopolyynes (the formula of which is R-(CºC)n-CN with R = H or Me) have been detected in the interstellar medium (ISM)[1] and on Titan.[2],[3] Our laboratory has been studying these compounds for several years by synthesizing them and studying their chemical or photochemical reactivity. However, despite the low number of atoms these compounds possess, their synthesis in laboratory still remains a scientific challenge. Thus, we will present the synthetic pathway for cyanopolyynes having two conjugated CºC triple bonds (n = 2), as well as their interesting reactivity with nucleophiles.[4],[5] However, even though we were able to synthesize these diynes, their superior counterparts (n = 3) stay elusive so far.
To solve this problem, we synthesized the bromocyanobutadiyne (5-bromopenta-2,4-diynenitrile ; Br-CºC-CºC-CN) and reacted it with different terminal alkynes under Cadiot-Chodkiewicz conditions.[6] Surprisingly, the corresponding cyanopolyynes were not obtained but more complex compounds, resulting from cascade reactions, were isolated. In particular, a diene was obtained stereoselectively when using monoacetylenic reactants. When using triisopropylsilylbutadiyne, a functionalized benzofulvene was obtained (Figure 1). The characterization of these unexpected products is based on X-ray crystallography, among other usual techniques. The mechanisms of formation of these products, which were studied both experimentally and theoretically, will be discussed.
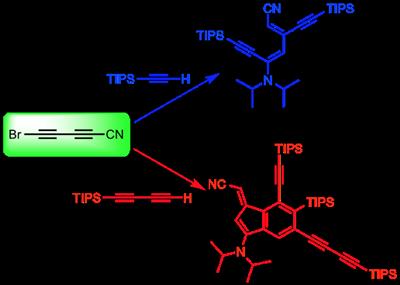
Figure 1. Reactivity of bromocyanobutadiyne with triisopropylsilylacetylene and triisopropylsilylbutadiyne
References:
[1] P. Thaddeus, M. C. McCarthy, M. J. Travers, C. A. Gottlieb, W. Chen, Faraday Discuss. 1998, 109, 121-135
[2] A. Coustenis, T. Encrenaz, B. Bézard, B. Bjoraker, G. Graner, G. Dang-Nhu, E. Arié, Icarus, 1993, 102, 240-260
[3] V. Vuitton, R. V. Yelle, V. G. Anicich, Astrophys. J. 2006, 647, L175-L178
[4] Y. Trolez, J.-C. Guillemin, Angew. Chem. Int. Ed. 2005, 44, 7224-7226
[5] a) N. Kerisit, L. Toupet, Y. Trolez, J.-C. Guillemin, Chem. Eur. J. 2013, 19, 17683-17686;
b) N. Kerisit, C. Rouxel, S. Colombel-Rouen, L. Toupet, J.-C. Guillemin, Y. Trolez, J. Org. Chem. 2016, 81, 3560−3567
[6] N. Kerisit, L. Toupet, P. Larini, L. Perrin, J.-C. Guillemin, Y. Trolez, Chem. Eur. J. 2015, 21, 6042-6047
Speaker information
Dr Yann Trolez. University of Rennes