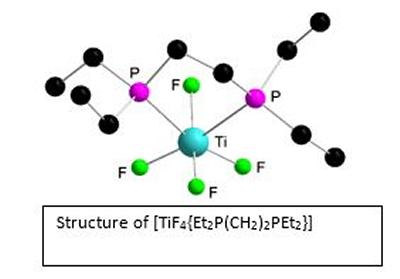
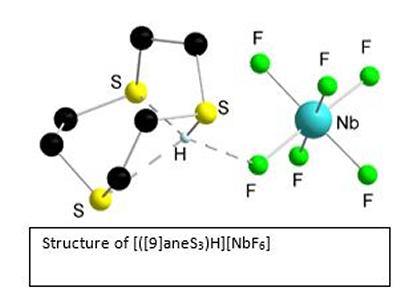
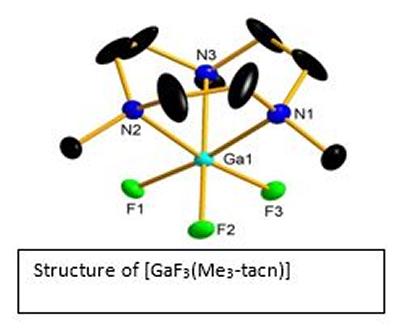
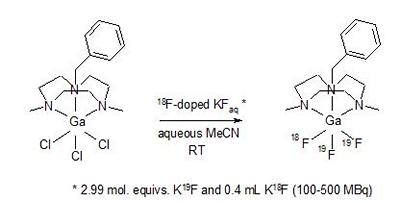
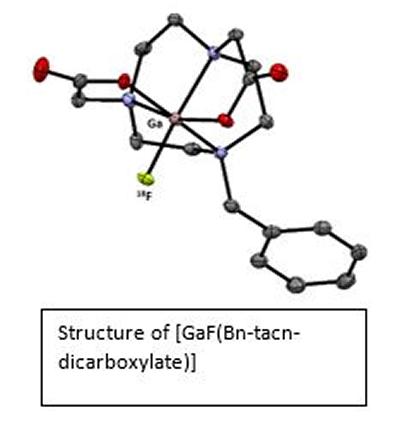
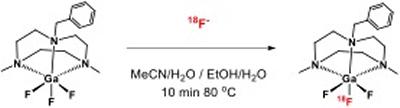
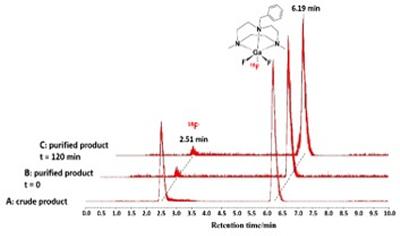
Research project: Reid: Metal Fluoride Coordination Chemistry
Until relatively recently metal fluoride coordination complexes, in contrast to the heavier halide counterparts, were rather neglected and little was known about their properties. However, it has become clear that the study of coordination complexes of inorganic fluorides is important and often the properties conferred on the acceptor centre by fluorine co-ligands are very significantly different – this is mainly a reflection of the strong M-F bonding imposing a different chemistry upon the metal centre.