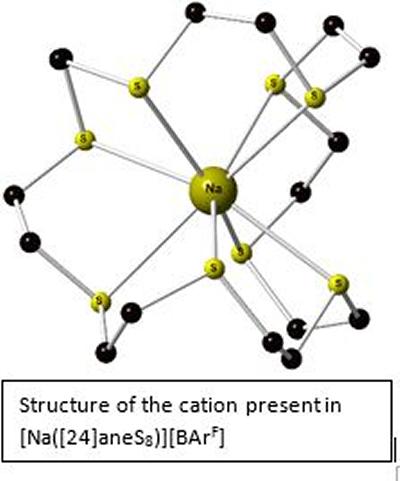
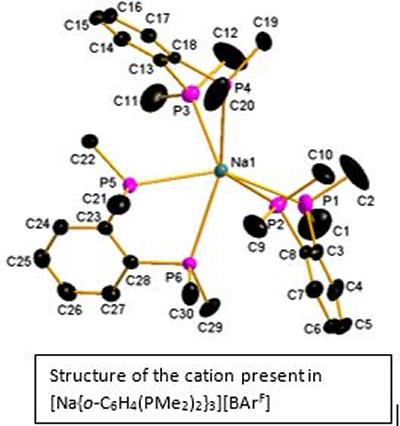
Research project: Reid: s-Block complexes with soft donor ligands
The neutral group 16 donor chalcogenoethers, ER2 (E = S, Se, Te) are generally regarded as soft, modest σ-donor ligands that form complexes readily with middle and late transition metals in low oxidation states. However, by controlling the reaction conditions and selecting the metal ion source carefully, new types of complexes containing soft chalcogenoether ligands with hard, early transition metal ions (including, for example, Ti, Zr, Hf(IV), V(IV), V(III), Nb(V), Ta(V), Cr(III), etc.) can be prepared. This opens up the opportunity to explore how these very unusual metal-ligand combinations affect the chemistry and reactivity within these unusual complexes. Similarly, neutral phosphine ligands, PR3, are ubiquitous in transition metal chemistry, owing to their capacity to tune the electronic and steric properties, and hence the reactivity, of the complexes, and to the strong σ-donor properties of the soft phosphine donor functions. This has led to wide utilisation of phosphine co-ligands in many transition metal reagents and catalysts. We have now extended this work to explore the chemistry of soft donor ligands towards alkali metal and alkaline earth cations.