Research project: Harrowven: Synthesis of Medium-Sized Rings, Biaryls and Triaryls
We have extended our use of radical additions to arenes to prepare medium-sized rings, including the tricyclic core found in the stegane family of natural products.
We have extended our use of radical additions to arenes to prepare medium-sized rings, including the tricyclic core found in the stegane family of natural products.
The method involves a new radical induced ring expansion strategy that is high yielding and can be carried out at normal dilution.
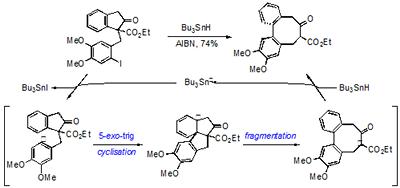
Scheme. A new approach to medium-sized rings and the carbon skeleton of the steganes.
The same tactic provides a useful entry to highly substituted biaryls and triaryls, which can be difficult to prepare using crossed-coupling methodologies. The reaction has been applied in a total synthesis of isoaucuparin, a natural product found in the sapwood tissue of Sorbus aucuparia.
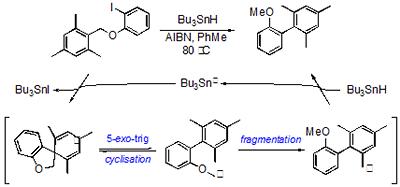
Scheme. A radical approach to biaryls.
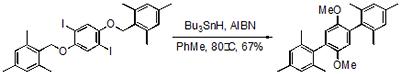
An efficient route to highly substituted triaryls.