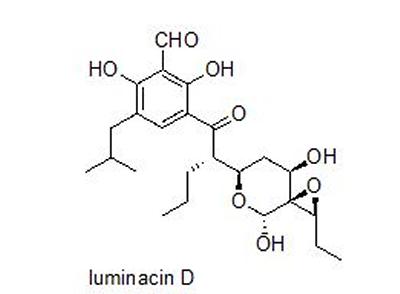
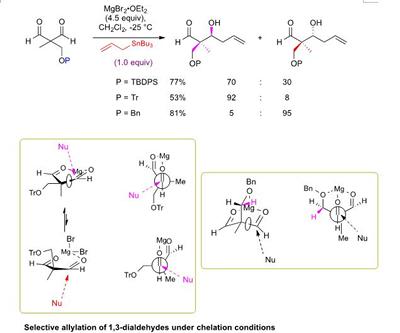
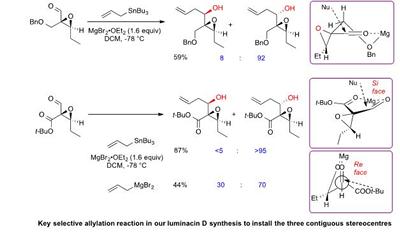
Research project: Linclau: The synthesis of luminacin D
Angiogenesis, the formation of new blood vessels, is a highly regulated process that plays an important role in a variety of functions such as wound healing and reproduction. Uncontrolled angiogenesis is associated with a variety of diseases such as rheumatoid arthritis, diabetic retinopathy (a major cause for blindness) and atherosclerosis. Tumor growth is also angiogenesis-dependent: the growth of new capillary blood vessels is essential for the tumor itself to grow. Furthermore, the new blood vessels in the tumor provide a gateway for tumor cells to enter the circulation, which results in the spread of the disease.