Trial Overview
Trial Team
Essential Trial Documentation
Title
Polatuzumab vedotin plus rituximab, ifosfamide, carboplatin and etoposide (Pola-R-ICE) versus R-ICE alone in second line treatment of diffuse large B-cell lymphoma (DLBCL).
Description
An open-label, prospective Phase III clinical study to compare polatuzumab vedotin plus rituximab, ifosfamide, carboplatin and etoposide (Pola-R-ICE) with rituximab, ifosfamide, carboplatin and etoposide (R-ICE) alone as salvage therapy in patients with primary refractory or relapsed diffuse large B-cell lymphoma (DLBCL).
The study will investigate the addition of polatuzumab vedotin (Pola) to rituximab, ifosfamide, carboplatin and etoposide (RICE), a standard chemotherapy treatment regimen versus treatment with RICE alone, in patients with primary refractory or relapsed diffuse large B-cell lymphoma (DLBCL).
Over 50% of DLBCL patients will not achieve a remission with standard second line salvage therapies. Therefore, there is an unmet medical need to optimise second line therapy in progressive and relapsed aggressive B cell non-Hodgkin lymphoma.
Polatuzumab vedotin is an antibody drug conjugate, targeted to the CD79b antibody expressed on a majority if B cell derived malignancies. In initial studies, polatuzumab vedotin has shown very promising response rates. The RICE regimen will be used as the comparator/standard arm. RICE is regarded as less toxic than some other regimens but still with comparable response rates.
This is the first study to assess the Pola-RICE combination and as such, as well as efficacy objectives, particular attention will be paid to safety objectives.
Objectives
Primary:
- The primary objective of this study is to investigate the following question in patients with relapsed or primary refractory DLBCL: Does salvage therapy with Pola-R-ICE improve event-free survival (EFS) compared to R-ICE alone?
Secondary:
The secondary efficacy objectives of the study are to collect data in order to evaluate whether the addition of polatuzumab vedotin to standard therapy R-ICE:
- Enhances the rate of metabolic complete response (CR) at the end of study treatment.
- Enhances the partial response (PR) rate and overall response rate (ORR) and decreases the progression rate and relapse rate.
- Enhances the duration of response, progression-free survival (PFS) and overall survival (OS).
- Impacts the mobilization of autologous CD34+ stem cells.
- Enhances the rate of patients proceeding to transplantation.
- Impacts the non-relapse mortality.
Further secondary objectives of the study regarding safety, protocol adherence, quality of life (QoL) and biology are to collect data in order to evaluate; adverse and serious adverse events, incidence and duration of neutropenia and thrombocytopenia Grade 4 CTC, rate of treatment-related deaths, number of second malignancies, cumulative and relative doses, and QoL, as well as to provide samples for translational research.
Trial Design
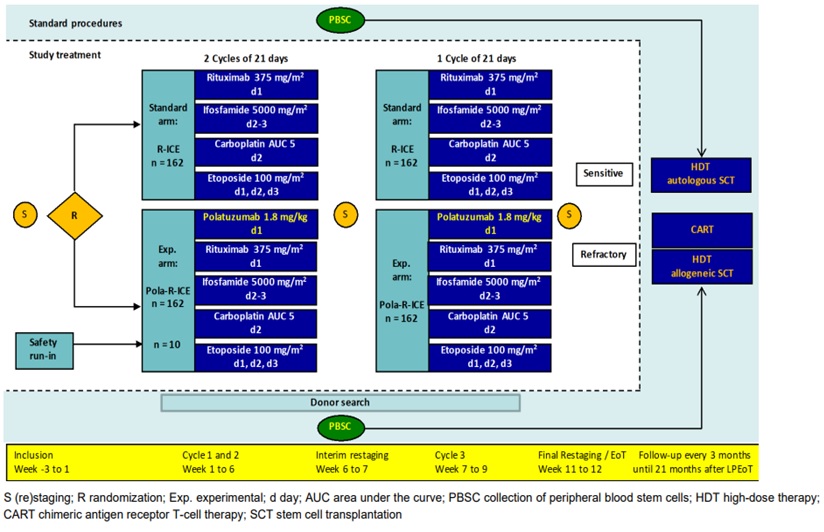
This is a multicentre, international, open-label, randomised phase III trial.
The study will have two treatment arms; Pola-RICE vs RICE and patients will be randomised on a 1:1 basis.
The study will aim to recruit 334 patients across 4 countries; Germany, Austria, Spain and the UK. 75 patients will be recruited from the UK.
Following informed consent, all patients will receive 3 cycles of chemotherapy; half the patients will receive Pola-RICE and half the patients will receive RICE. Patients will then be followed up (3 monthly visits) for at least a further 21 months. Therefore, each patient will be involved in the study for a minimum of 24 months.
Trial Status
Open to recruitment
Population
Patients aged 16 years and over with relapsed or primary refractory DLBCL.

The study is an investigator-initiated trial with financial support by F. Hoffmann-La Roche Ltd. The trial is endorsed by Cancer Research UK (award ref CRCET\100004) and NIHR adopted.
Senior Trial Manager:
Joshua Caddy
Trial Manager:
Tracey Mason
Contact Information for trial queries:
Email: polarice@soton.ac.uk
Phone: 023 8120 5154
SAE Reporting:
PV is being performed by ZKS Leipzig
Email: pharmocovigilance@zks.uni-leipzig.de
Phone: +49 341 97 16129
Fax: +49 341 97 16278
Study Documents:
Pola-R-ICE – Study Contacts and Information v1 21 Mar 2022
Pola-R-ICE – UK Specific Protocol Appendix v2 01 Mar 2022
Pola-R-ICE – Protocol v4, 28 Aug 2022
Pola-R-ICE – Financial Disclosure v3 19 Jan 2021
Pola-R-ICE – Patient Identification Log v1 19 Jan 2021
Pola-R-ICE –Site Delegation Log v2.1 18 Jun 2021
Pola-R-ICE –Site Training Log v2.1 18 Jun 2021
Patient Documents
Pola-R-ICE –GP Letter v2 20 Dec 2021
Pola-R-ICE –Informed Consent Form v2 20 Dec 2021
Pola-R-ICE –Optional translational samples consent form v2 20 Dec 2021
Pola-R-ICE –Optional Translational samples Patient Information Sheet v2 20 Dec 2021
Pola-R-ICE –Patient Information Sheet v2 20 Dec 2021
Pola-R-ICE –Pregnancy follow up consent form v2 20 Dec 2021
Pola-R-ICE –Pregnancy Information Sheet v2 20 Dec 2021
Pharmacy Documents
Pola-R-ICE –Pharmacy Manual v3 18 Oct 2022
Pola-R-ICE –IMP Shipping Request v5
Pola-R-ICE –Patient DAF Carboplatin v5 24 May 2022 UK
Pola-R-ICE –Patient DAF Etoposide v5 24 May 2022 UK
Pola-R-ICE –Patient DAF Ifosfamide v5 24 May 2022 UK
Pola-R-ICE –Patient DAF Polatuzumab v5 24 May 2022 UK
Pola-R-ICE – Patient DAF Rituximab v5 24 May 2022 UK
Pola-R-ICE - IB Polivy v14, Oct 2022
Pola-R-ICE - IB Rituximab v28 Jun 2022
Laboratory Documents
Pola-R-ICE –HMDS Sample Shipment Form v2 20 Oct 2022
Pola-R-ICE –HMDS Sample Dispatch Log v1 05 Oct 2022
Pola-R-ICE –Laboratory Manual v1 15 Sep 2021
SAE Documents
Pola-R-ICE –SAE report form v2 15 Nov 2022