Trial Overview
Trial Team
Essential Trial Documentation
Title
An open-label, multicentre, randomised phase II Pembrolizumab in combination with R-ICE chemotherapy in relapsed/refractory diffuse large B-cell lymphoma.
Description
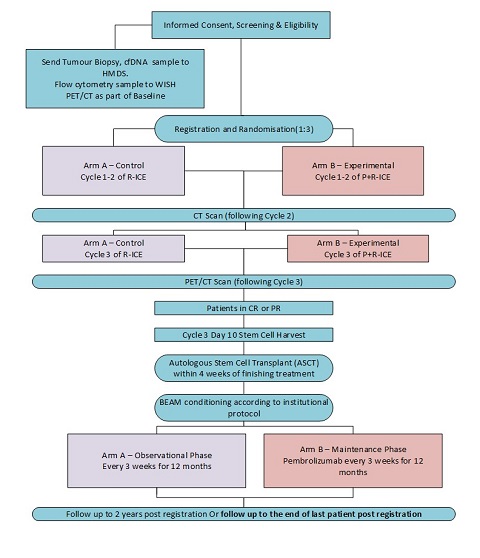
The study has two treatment arms to which participants will be randomised on a 3:1 basis to the experimental arm.
The control arm (Arm A) will be R-ICE for 3 cycles followed by an autologous stem cell transplant (for patients in a CR or PR on the post treatment PET-CT scan). The experimental arm (Arm B) will consist of P+R-ICE for 3 cycles followed by an autologous stem cell transplant (for patients in a CR or PR on the post treatment PET-CT scan) and maintenance Pembrolizumab every 3 weeks for one year. All patients will be randomised at study entry and will be stratified by relapse within 12 months or > 12 months of first line therapy.
- UK Sites – 9
- Recruitment target – 65
- Recruitment period – 24 months
Objectives
Primary:
- To establish the event-free survival at 1 year in patients treated with P+R-ICE.
An event is defined as any of the following:
- Progression / relapse of lymphoma
- Stable disease at 3 cycles of therapy
- Commencement of any unplanned non-protocol treatment for lymphoma
- Death from any cause
Secondary:
- To examine the longer-term (2 year) efficacy of P+R-ICE
- To assess whether the addition of pembrolizumab has an impact on the ability to harvest sufficient peripheral blood progenitor cells for autologous stem cell transplant
- To assess the number of patients achieving CR following treatment with P+R-ICE
- To document the safety and toxicity profile of P+R-ICE
- To document the anti-tumour activity of P+R-ICE in patients with relapsed or refractory DLBCL
Tertiary:
- To correlate clinical outcomes with gene expression analysis of PD-1, PD-L1, PD-L2 and other immune signatures from primary tumour material.
- To correlate clinical outcomes with immunohistochemical expression in tumour material of PD-1, PD-L1, PD-L2 and other markers in both tumour and microenvironment
- To correlate clinical outcomes with expression of PD-1, PD-L1/L2 on peripheral blood T-cells. Subdivision of T-lymphocyte sub-sets
Trial Design
Trial Status
Open to recruitment.
Population
Population over 18 years old with relapsed/refractory diffuse large B-cell lymphoma.
Funder
This trial is funded by Merck Sharp & Dohme (MSD) known as Merck & Co.
Any additional costs will be covered by SCTU.
Senior Trial Manager:
Josh Caddy
Trial Manager:
Amber Cole
Trial Monitors:
Parys Hatchard
Aleksandra Kusinska
Data Manager:
Zoe Konn
Clinical Trials Data Coordinator:
Oli Seymour
Contact Information for trial queries:
Email: PRICEtrial@soton.ac.uk
Phone: 023 8120 5154
SAE Reporting:
Email: ctu@soton.ac.uk
P+R-ICE - Main Patient Information Sheet _v3 26Jan2022
P+R-ICE - Pregnant Partner Patient Information Sheet v2_24Nov2021
P+R-ICE - Informed Consent Form v2_24Nov2021
P+R-ICE - Pregnant Partner ICF v1 20Aug2021
P+R-ICE - GP letter v2_24Nov2021
P+R-ICE - GP Pregnant Partner Letter v1_24Nov2021
P+R-ICE - Site Delegation Log v1 04-Jan-2022
P+R-ICE - Screening Log v1 02July2021
P+R-ICE - Master Patient List v1 02 July 2021
P+R-ICE - Site Training Log v1 12-Mar-2021
P+R-ICE - Site Visit Log v1 12-Mar-2021
P+R-ICE - HMDS Tissue Samples Dispatch Log v0.1 19-March-2021
P+R-ICE - HMDS Sample Shipment Form v0.1 22-March-2021
P+R-ICE - WISH Sample Shipment Form v0.1 07-May-2021