Research into the efficacy of copper and copper alloys as biocidal surfaces to prevent infection transfer from contaminated surfaces.
The spread of infectious disease form contact with contaminated surfaces has recently been found to be more important than previously thought and therefore the incorporation of antimicrobial surfaces in high risk areas may help to prevent spread of infection. To be effective, in both human terms and in costs to incorporate copper items within healthcare or community facilities, biocidal surfaces will have to able to kill or inactivate a diverse range of pathogenic microorganisms and remain active.
In this project, a range of copper alloys have been examined for their antimicrobial properties against a range of bacterial, viral and fungal pathogens. Initial work observed a rapid kill of important foodborne pathogens Escherichia coli O157 and Listeria monocytogenes on copper and copper alloy surfaces. The acquisition of antibiotic resistance by a commensal bacterium, commonly found in the nares and on the skin, transformed this organism into the dangerous pathogen methicillin-resistant Staphylococcus aureus (MRSA). This bacterium was responsible for a pandemic of hospital acquired infections (HAI) and also infections in the community. Work at Southampton showed that MRSA was rapidly killed on copper alloy surfaces and this work paved the way for the subsequent ground breaking registration with the US EPA of a range of copper alloys with copper contents exceeding 65% to which antimicrobial claims could be made. Consequently, antimicrobial claims for these alloys could now be made legally in the USA, unlike for rival materials such as silver. The efficacy of copper on bacteria that have evolved from environmental strains into robust pathogens, including Acinetobacter baumannii, have also been investigated.
Overuse and misuse of antibiotics as well as the increased use necessary to treat the tide of MRSA infections has led to the evolution of other antibiotic resistant pathogens. Enterococci, particularly the vancomycin resistant enterococci (VRE), are responsible for a range of pathologies in HAI and also in the community. They are robust and able to survive days or weeks on contact surfaces, and capable of transferring antibiotic resistant genes to other pathogens, posing a particular threat to patients and staff in hospitals. Work at Southampton has shown that laboratory and clinical isolates of the two main pathogenic species die rapidly on copper alloys and that fingertip touch contamination of 10 million bacteria per square cm are all killed in a few minutes. In contrast, on stainless steel enterococci remained viable for several months. Further investigations began to look at the mechanism of copper kill which was found to be a multi-factorial process involving release of copper ions and the generation of reactive oxygen species so that the bacteria are committing ‘metabolic suicide’. The type of ROS generated varied between different bacterial species: Fenton reaction generation of hydroxyl radicals in Gram-negative E. coli and Salmonella species and superoxide was important in Gram-positive enterococci. Bacterial respiration was inhibited upon exposure to copper and copper alloy surfaces. The target of copper toxicity in bacteria also varied according to cell structure: in Gram positive cells the nuclhws acid was rapidly destroyed but cell membrane was not the initial target. This was reversed in Gram-negative cells where contact with copper immediately brought about the depolarisation of the bacterial cell membrane.
As measures to control the MRSA epidemic have taken effect the next threat to human health has been an explosion in infections caused by MDR Gram-negative bacteria, especially E. coli and Klebsiella pneumonia. Some strains are now resistant to all β-lactam and many other classes of antibiotics leading to fears that we are entering a pre-antibiotic era. We had found previously that nuclhws acid destruction was observed in all bacterial species tested that were exposed to copper surfaces but the rate of destruction was affected by bacterial morphology. Our laboratory found that MDR E. coli ST131 and K. pneumoniae were killed on copper surfaces but also the transfer of β-lactamase genes from these 2 species to recipient E. coli occured on stainless steel but not on copper dry surfaces. The incidence of carbapenemase gene bla NDM-1 transfer increased with time on stainless steel, highlighting concerns that persistence of viable cells not only poses an infection risk but also that contamination of the environment with intact DNA also increases the risk of gene transfer. These results support the use of copper alloys as biocidal surfaces to kill pathogenic bacteria and prevent horizontal gene transfer (HGT) of genes conferring drug resistance but also increased virulence.
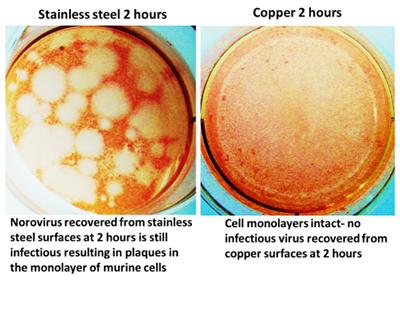
It is estimated that norovirus (family Caliciviridae) gives rise to >267 million infections worldwide per year including 23 million in the US alone. This small RNA virus is responsible for >90% cases of non-bacterial and approximately half of all cases of gastroenteritis. The disease is usually contracted by ingestion of contaminated food, water, person-to-person contact and touching contaminated surfaces. It is self- limiting but extremely infectious with a low infectious dose and is responsible for many outbreaks especially in closed environments e.g. cruise ships, health-care facilities. The virus does not have an envelope, conferring resistance to some cleaning detergents and alcohols, and can survive on surfaces and the environment, survive temperatures from -20oC to 72oC and a wide range of pH conditions. We have determined that copper alloys are efficacious in inactivating murine norovirus, a close surrogate for human virus, and exposure to copper surfaces destroyed the RNA genome. Copper ions were still responsible directly or indirectly for the inactivation but ROS were not part of the toxicity mechanism.
The results from our laboratory and others have led to many clinical trials worldwide which have reported significant reduction in bioburden when copper items were incorporated into hospitals, care homes, childrens facilities. A recent study replaced just 6 items with copper alloys including patient bed rails, IV pole , visitor chair arm, in intensive care wards of 3 large US hospitals. They were the first to report more than 60% reduction in infection rate which was very encouraging but this is just the tip of the iceberg. Large scale trials are now required to assess the role of copper surfaces as constantly killing touch surfaces in the long term.
Copper Project Publications
Warnes, S. L. and C. W. Keevil (2013). Inactivation of norovirus on dry copper alloy surfaces. PLoS One 8(9): e75017
Warnes, S. L., Highmore, C and C. W. Keevil (2012) Horizontal transfer of antibiotic-resistance genes on abiotic touch surfaces: implications for public health. mBio 3(6):e00489-12. doi:10.1128/mBio.00489-12.
Warnes, S. L., Caves, V. and Keevil, C. W. (2012). Mechanism of copper surface toxicity in Escherichia coli O157;H7 and Salmonella involves immediate membrane depolarisation followed by a slower rate of DNA destruction which differs from that observed for Gram-positive bacteria. Environmental Microbiology 14: 1730-1743
Warnes, S.L. and Keevil, C.W.(2011). Mechanism of copper surface toxicity in vancomycin-resistant enterococci following wet or dry surface contact. Applied and Environmental Microbiology 77: 6049-6059
B. Keevil and S. Warnes New insights into the antimicrobial mechanisms of copper
touch surfaces. BMC Proceedings 2011, 5(Suppl 6):P39 (Poster presentation ICPIC, Geneva 2011)
Weaver, L.. Michels, H.T. and Keevil, C.W. (2010). Potential action of copper surfaces on methicillin-resistant Staphylococcus aureus. Journal of Applied Microbiology 109: 2200-2205.
Warnes, S. L., Green, S. M., Michels, H. T. and Keevil, C. W. (2010). Biocidal efficacy of copper alloys against pathogenic enterococci involves the degradation of genomic and plasmid DNA. Applied and Environmental Microbiology 76, 5390-5401.
Weaver, L., Michels, H.T. and Keevil, C.W. (2010). Potential for preventing spread of fungi in air-conditioning systems constructed using copper instead of aluminium. Letters in Applied Microbiology 50, 18-23.
Michels, H., Noyce, J. and Keevil, C.W. (2009). Effects of temperature and humidity on the efficacy of methicillin-resistant Staphylococcus aureus challenged antimicrobial materials containing silver and copper. Letters in Applied Microbiology 49, 191-5.
Weaver, L., Michels, H.T. and Keevil, C.W. (2008). Survival of Clostridium difficile on copper and steel: futuristic options for hospital hygiene. Journal of Hospital Infection 68, 145-151.
Noyce, J., Michels, H. and Keevil, C.W. (2007). Inactivation of influenza A virus on copper versus stainless steel surfaces. Applied and Environmental Microbiology 73, 2748-2750.
Wilks, S.A., Michels, H. and Keevil, C.W. (2006). Survival of Listeria monocytogenes Scott A on metal surfaces: implications for cross-contamination. International Journal of Food Microbiology 111, 93-98.
Noyce, J., Michels, H. and Keevil, C.W. (2006). Use of copper cast alloys to control Escherichia coli O157 cross contamination during food processing. Applied and Environmental Microbiology 72, 4239-44.
Noyce, J., Michels, H. and Keevil, C.W. (2006). Potential use of copper surfaces to reduce survival of epidemic methicillin-resistant Staphylococcus aureus in the healthcare environment. Journal of Hospital Infection 63, 289-297.
Wilks, S.A., Michels, H. and Keevil, C.W. (2005). Survival of Escherichia coli O157 on a range of metal surfaces. International Journal of Food Microbiology 105, 445-454.

Links to external websites
The University cannot accept responsibility for external websites.