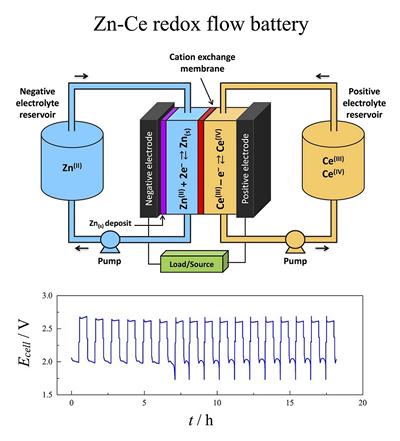
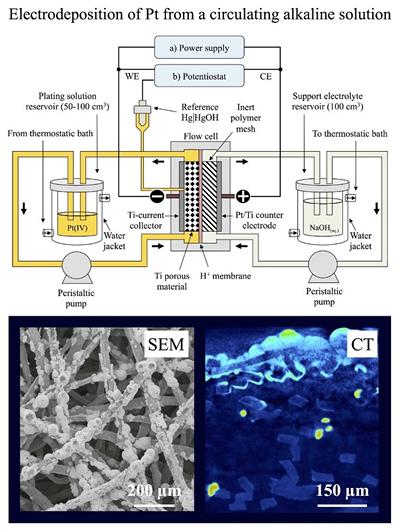
Research project: Redox flow cells batteries: zinc - cerium
Energy storage is essential in view of the rapidly growing demands for low cost energy based on sustainable resources. Redox flow batteries have the capacity to store the energy generated during periods of larger production and low demand.