NAMRIP funding in 2017 and 2020 started the route to commercialize novel ureteric stents
NAMRIP pump-priming support in 2017 for ureteric stents produced pre-clinical data and clinical collaborations, leading to larger grants and market research on the route to commercialization. A later grant from NAMRIP (and the NIHR Southampton Biomedical Research Centre) in 2020 pushed the work further en route to commercialisation.
Background: the problem
Ureteric (or ureteral) stents are medical devices used by clinicians as a temporary measure to retrieve urine drainage when it is compromised as a result of kidney stones, tumours etc. Ureteric stents are tubes that are a few centimetres long, which are placed into the ureter with certain design characteristics, such as curled ends (one in the kidney and one inside the bladder) to anchor the stent in place.
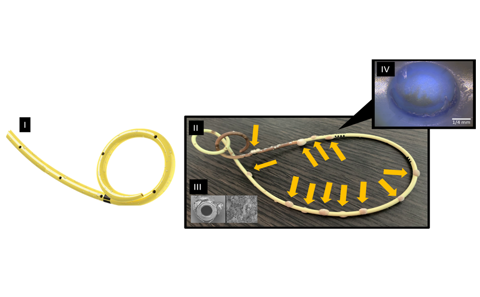
The design characteristic that is of particular interest in this study consists of rows of holes in the side of the stent to enable urine to bypass the obstruction [shown by a partial schematic in Figure (I)]. However, shortly after the stent is implanted, these holes can become covered by crystals and bacterial deposits (such as encrustation and biofilm) [Figures (II) to (IV)]. This can lead into urinary tract infection (UTI), often requiring antibiotic treatment. Furthermore, these deposits block the stent and reduce its performance. Clinicians recognise this as a failure and as result they may resort to premature removal and replacement of the stent. This can happen multiple times following a single initial stent implant.
Over the years, many novel materials, coatings, and designs have been suggested to address this challenge, but encrustation and biofilm formation are still a major problem for stents, in part because the mechanism behind these failures is not well understood.
Figure (I) shows a partial schematic of an unused stent. Figure (II) is a photograph of a retrieved stent that shows particle deposition (encrustation and biofilm formation) as well as discolouration. Encrustation is referring to the crystallisation and biofilm formation is referring to a community of bacteria on a surface surrounded by a glue-like matrix as seen on Figure (III). Figure (IV) shows EDIC (Episcopic Differential Interference Contrast) microscopy of a conventional side-hole of a ureteric stent retrieved from a patient, the hole being in the initial phase of deposition.
Funding from NAMRIP
In 2017, NAMRIP began funding Dr Ali Mosayyebi and his team from Southampton University to conduct research to:
· understand the mechanism behind the particle deposition (encrustation and biofilm formation) on ureteric stents;
· conduct pre-clinical (i.e. bench test) studies of novel stent designs that would suffer from reduced particle deposition;
· establish collaboration with clinical surgeons; and
· gain better understanding of the real-world challenges ahead of translating the technology.
The team used fluid dynamics to understand the mechanism behind particle deposition in the urinary tract and governing flow processes. This has led into a patented ureteric stent design with novel side-holes. The technology is successfully tested in a benchtop setting, investigated a pre-clinical safety study, and currently being developed to go through a first-in-human clinical investigation. To ensure patients benefit from the technology, the team is establishing a spin-out (called Sooba Medical) as a commercial vehicle to lead the routes market.
Detail
The success of the 2017 NAMRIP pump-priming project allowed the team to publish and engage with the clinical community at international congresses, and this in turn led to further translational steps of the technology via market research (by joining ICURe programme), performing a pre-clinical (i.e. animal test) study to establish commercial strategies for commercialising the technology, and raising the next phase of the funding to perform first-in-human clinical study through NIHR i4i PDA.
In 2020, once again NAMRIP was able to help by providing a funding to take another crucial step in further validating the technology and support the upcoming clinical investigation, by providing funds in a joint collaborative funding programme between NAMRIP and the NIHR-funded Biomedical Research Centre.
This 12-month project aimed to develop a series of primary and secondary output (listed below) to support the next phase of the clinical adaptation of the patented ureteric stent technology (ureteric stent) and strengthening the output of the NIHR i4i grant proposal.
The primary anticipated outcomes of the project were:
- Developing a Patient Information Sheet and Informed Consent Form (PIS-ICF) that will be used for human trials;
- Finalising a patient recruitment strategy and materials (advertisements, PIS and ICF) through discussions with PPI contributors.
The secondary outcomes were going to be:
- Identify 1 to 2 lay co-applicants to join the NIHR i4i application team;
- Establish a strategy to involve PPI contributors in execution of NIHR activities.
While the team was working closely with PPI (patient and public involvement) representatives (i.e. patients and carers), given the impact of these outcomes on the upcoming clinical investigations, they were able to join forces with SCTU (Southampton Clinical Trials Unit) and develop the first phase of the ethics approvals, which was going to not only allow them to understand patients' experience with having stents, but also collect actual urine and stent samples and analyse them with the view of finding the dominant bacterial species present in these samples. Thanks to the support from the funding team, they were also able to go one step further and not only acquire ethics but recruit the first cohort of patients and collect their samples. Additional outputs include:
- PIS and ICF developed;
- Recruitment strategy/approach to reach as many participants as possible established;
- Lay PPI reps to join the NIHR project team were identified;
- The strategy in involving PPI reps to contribute to the execution of the NIHR project was established;
- A complete clinical protocol to collect real-life patient data was created;
- A detailed flow chart of data flow all the way from screening to recruitment and analysing data was created;
- Ethics submitted and successfully acquired (IRAS: 305000);
- First NHS site was successfully opened;
- First cohort of patients were recruited;
- Patient samples were successfully collected;
- First raw microbiology data, including microscopy images and cell culture of stent and urine samples were completed [Figure (IV)].
Dr Mosayyebi said: 'The grant from Global-NAMRIP and BRC enabled us to take a huge step towards creating all the relevant documents to support upcoming clinical investigations, acquiring ethics, and successfully recruiting first patient cohort to provide real world data of conventional stents that are currently used in the market, which could be used as a control database for the NIHR project. Additionally, the learnings from this work provided us with a head start in developing the relevant documentations for the NIHR project. Overall, this study has:
- allowed us to develop a specific laboratory protocol in analysing urine and ureteric stent samples.
- identified new opportunities in better understanding the encrustation and biofilm formation on ureteric stents.
- further strengthened the output of the NIHR project by allowing us to create a more comprehensive protocol and have a more meaningful analysis of the novel ureteric stent. This will support us towards successful delivery of the NIHR project and getting one step closer in bringing our novel technology to the real world.'
He continued: 'I am very grateful to Professor Leighton and NAMRIP for recognising the potential of this work at an early stage, and supporting it through pump priming, and then again at a later stage with the NAMRIP/BRC joint funding. The continued support which enabled us to take meaningful steps closer in translating our technology to the real world and impacting not only in patients' quality of life but also in help the NHS both financially and in freeing up hospital beds.'