Project summary
Neisseria gonorrhoeae, the gonococcus, is the causative organism of the sexually transmitted disease (STD) gonorrhoea. The disease has affected humans for centuries and gonorrhoea is a serious infection for both men and women. For men, the infection is a painful condition, but for women it can be present without symptoms and the outcomes can be more serious, e.g. pelvic inflammatory disease, ectopic pregnancy, infection of the Fallopian tubes and infertility. Current estimates are for ~106 million new cases of gonorrhoea worldwide, annually out of a total of ~400 million cases of notified STDs of all causes.
In the past, gonorrhoeae was treated readily with antibiotics; however, N. gonorrhoeae has developed resistance to nearly all of the antibiotics used for treating gonorrhoea such as the sulfonilamides, penicillin, tetracycline, and fluoroquinolones (e.g. ciprofloxacin). There is now only one last effective class of antibiotics, the cephalosporins, to treat this STD infection. Unfortunately, we are currently seeing the emergence of cephalosporin-resistant gonorrhoea worldwide, including in the United States and the United Kingdom.
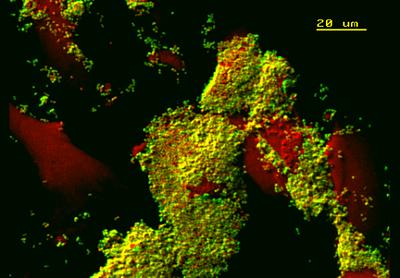
In the picture, as the gonococci (in green) interact with human endometrial cells from the female reproductive tract (in red), they appear to show a propensity for forming ‘sheets’ of attachment typical of biofilms.
Our work
Our approach to combat antibiotic resistance in the gonococcus is to focus on developing vaccines, a strategy that has languished for decades due to the success of antibiotic treatment. However, there is now a new urgency to develop protective vaccines against this STD. We have been working on several proteins that are found in the membrane of the bacterium and that are potential vaccine candidates.
Our process involves making these proteins in the laboratory and examining how they produce protective antibodies that can kill the bacteria and also prevent them sticking to human cells during infection. To produce new vaccines we need information about the chemical and physical structure of these proteins, including high-resolution X-ray crystallography.
About the team
Dr Myron Christodoulides (Reader in Molecular Bacteriology/Microbiology) is the NAMRIP Pump Priming project lead. His background is studying infections caused by various bacteria and developing vaccines for animal and human use. His team in the Faculty of Medicine consists of postdoctoral research fellows and students, who are engaged in a variety of projects studying antimicrobial resistant bacteria. There is also a strong association with clinical ophthalmologists in a program to investigate bacterial infections of the eye.
Our collaborators on this project are Dr Ivo Tews, an expert in protein crystallography and facility manager of the Macromolecular Crystallisation Facility, Centre for Biological Sciences aty the University; and Dr Maurits de Planque, ECS, Faculty of Physical Sciences and Engineering.
The project will focus on a technique called Random Microseed Matrix-Screening (rMMS) for making crystals of vaccine proteins and expertise is provided Dr de Planque together with technical assistant Chris Holes. The work will lay a solid foundation for a ‘structure-based’ approach to vaccine design for combatting gonorrhoea.
Engagement
Dr Christodoulides presented recently at a CafeScientifique meeting (May, 2016) in which he talked about ‘Understanding bacteria: friends and foes’, in which he discussed the challenges of antibiotic resistance.