Research uncovers a new way of making chiral catalysts
Scientists at the University of Southampton have discovered a new way to create one hand of a chiral molecule by using a mechanical bond as a catalyst.
Chiral molecules are two molecules that are mirror images of each other but not identical – much like the left and right human hands. When such molecules are used in pharmaceutical drugs, each “hand” reacts differently in the human body. In some cases whilst one hand treats the illness, the other side can be toxic so being able to make only one hand is extremely important.
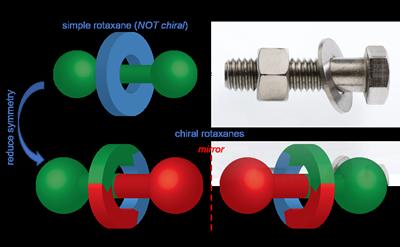
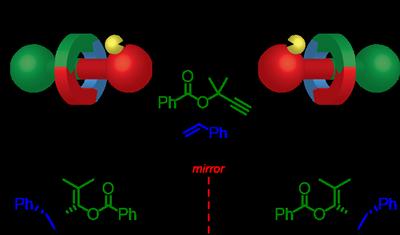
Rotaxanes consist of a ring shaped molecule wrapped around a dumbbell shaped axle (like a washer on a bolt). The ring and axle are held together by what we call a mechanical bond, as opposed to chemical bonds that usually connect atoms to form molecules.
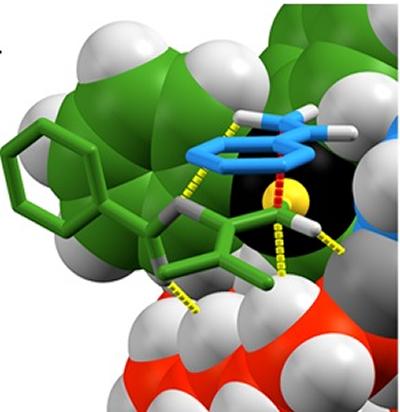
In a new study, published in the journal Chem, Professor Steve Goldup from the University of Southampton’s School of Chemistry made use of these mechanical bonds to create chiral molecules. Professor Goldup and his team synthesised a chiral rotaxane that could bind to gold atoms; the gold atoms were then used to catalyse a simple chemical reaction.
Professor Goldup said: “Chiral molecules, and how to make a single hand of them have been investigated since the birth of chemistry. Chiral rotaxanes have not been used in these studies to any extent as until recently they were very hard to make in one mirror image form. My group developed a very simple, general concept to make chiral rotaxanes as a single hand. This means we can now start investigating what problems they can help us solve in chemistry, biology and materials science.”
Chiral rotaxanes are an ideal environment for catalysts as the structure of a ring wrapped around the axle creates a tightly packed space for a reaction to take place in. The Southampton team were able to use one hand of the mechanical bond to selectively produce the target molecule and the opposite hand to produce the mirror image of the target.
Professor Goldup concluded, “We think the future is very bright for chiral rotaxanes now we can make them. As well as looking into further possibilities for catalysis and broader studies of molecules, we are investigating the how they can be used to potentially produce brighter, longer lasting, low computer power displays.”
The research was funded by the European Research Council.