Bone collected by our study will undergo mechanical testing and imaging of its structure with the aim to investigate changes in diseased bone that lead to fracture.

Reference Point Indentation
This research is investigating tools that have clinical potential for assessing bone quality and fracture risk, principally reference point indentation (RPI). RPI (the Biodent device from ActiveLife Scientific) is being applied to bone from clinical studies (OStEO and COASt) and a tissue bank to compare bone of high and normal fracture risk.
The technique involves a probe that cyclically indents into the bone relative to a reference probe, with higher indentation distance implying reduced bone quality. The aim is to investigate if the technique can differentiate between bone depending on its fracture risk (e.g. between fractured and non-fractured bone from OStEO and COASt). Additionally, it aims to be seen if RPI can measure bone quality with comparison to laboratory techniques (fracture toughness) which is distinct from bone quantity and existing technique (bone mineral density through DEXA).

Fracture Toughness
Bone quality relates to the structural and mechanical properties of the bone. Fracture toughness is a property that quantifies resistance to fracture and is therefore a relevant measure of the mechanical aspect of bone quality. However, there are difficulties in assessing fracture toughness in small bone samples as are tested in this research. For this reason, a novel technique that assesses the damage propagation through 'Whitening Front Tracking' has been previously developed in our laboratory.
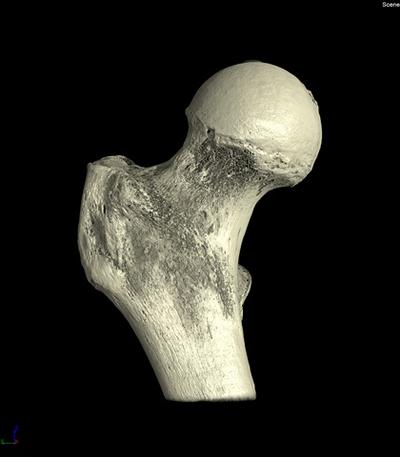
Micro-Computed Tomography
In addition to clinical risk factors, bone quantity through bone mineral density as measured by DEXA (Dual Energy X-Ray Absorptiometry) is used clinically for assessment of osteoporosis and increased fracture risk. For this reason, DEXA scans and risk factors will be assessed for the participants of the OStEO study, but for the bone from the tissue bank, this clinical assessment is not feasible. Instead, micro-computed tomography, a laboratory tool (equipment at the μ-VIS centre), is being developed to emulate the clinical assessment.
Additionally, the system can image the bone at high resolution so can be used to assess structural properties and their alterations with increased fracture risk, another aspect of bone quality.