Biomedical Devices
Details
The figure below depicts our first results for successfully crimping a model of the Edwards Lifesciences Sapien XT aortic valve. The model includes a metallic frame, the skirt and the three leaflets held in place by clips and a complex set of constraints.
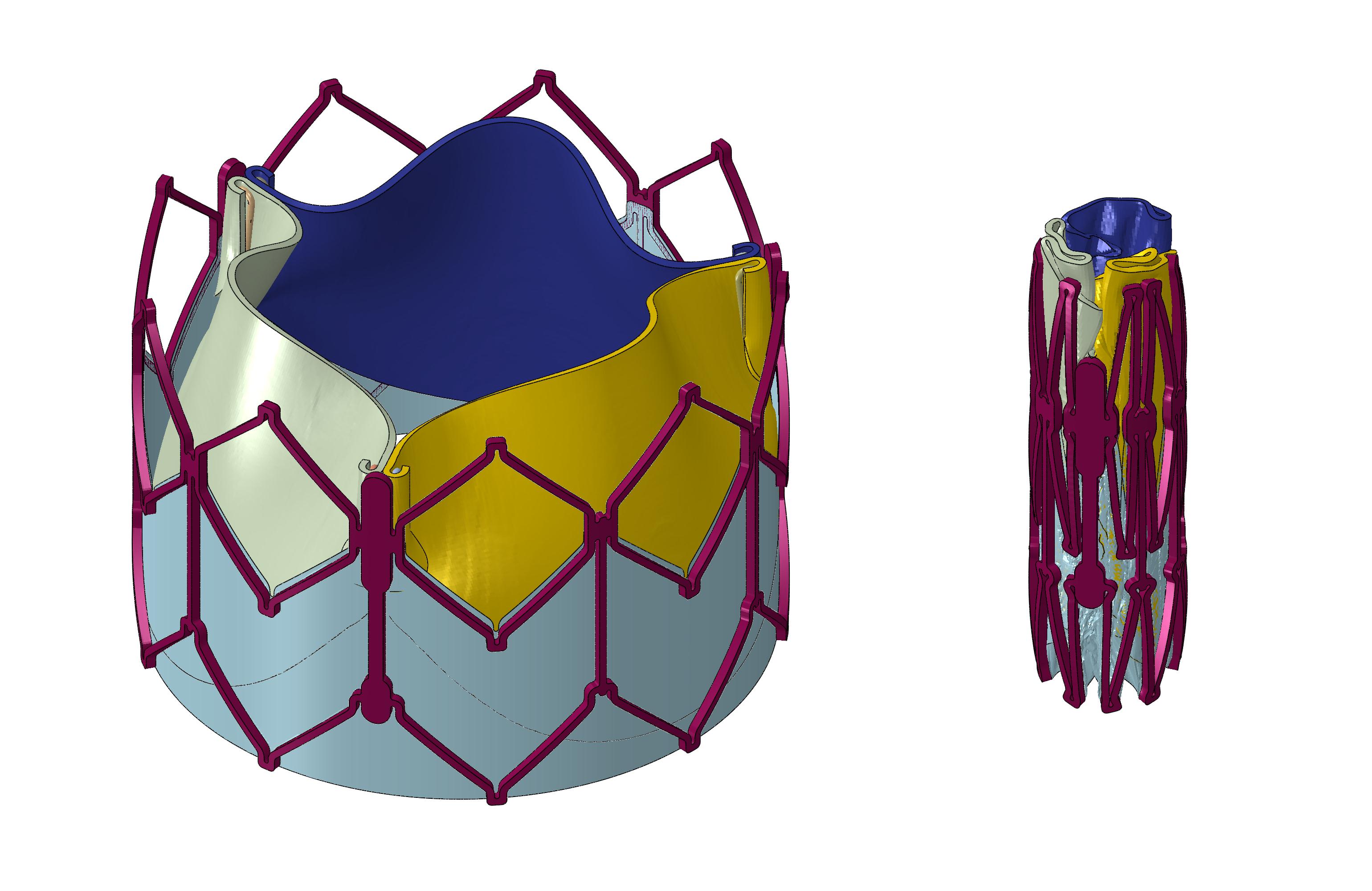
Subject to confidentiality.
The following animation depicts how a stepped balloon out performs undersize and oversize balloons in a complext bifurcation case.
This project is investigating stent malapposition and longitudinal deformation in patient specific coronary artery segments.
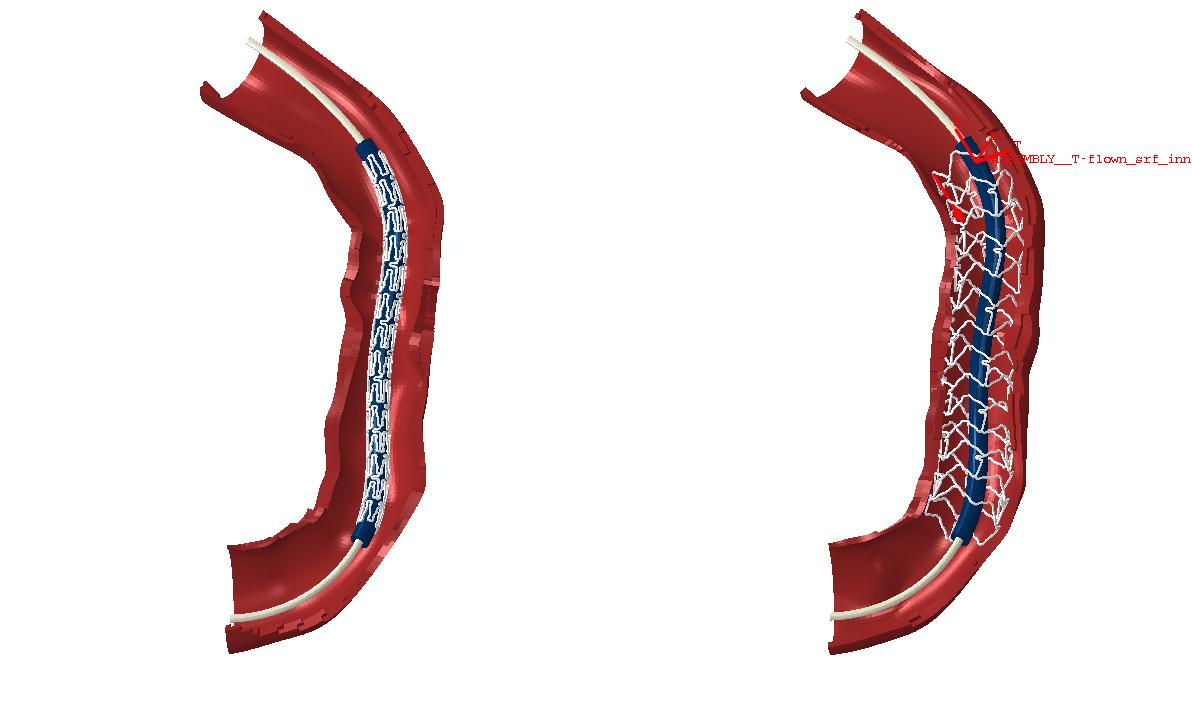
This has been the main focus of recent work. Shape control of coronary stents is being used to perform multi-objective, multi-disciplinary optimisation. Simulations are performed for families of designs that undergo deformation through balloon inflation (testing radial strength and avoidance of recoil); bending (testing for flexibility); drug release (aiming for good drug distribution in surrounding tissue) and flow disturbance (assessing how wall shear stress patterns are affected).
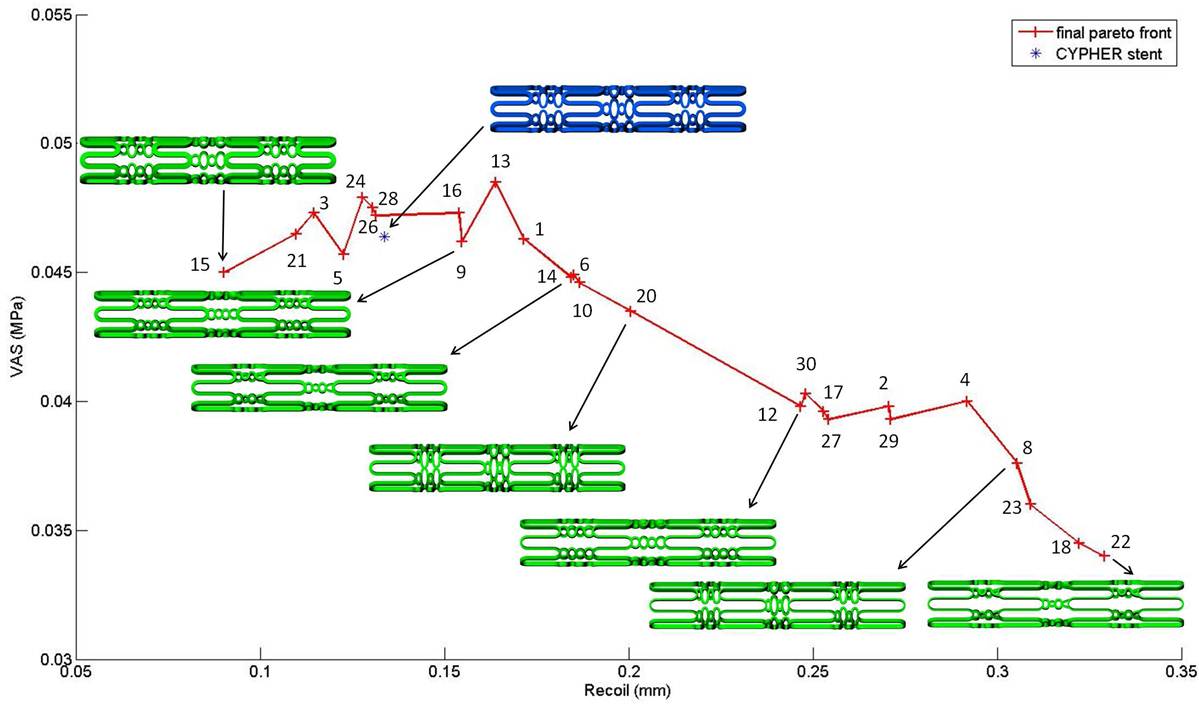
Measures of performance are combined in multi-objective optimisation that yields Pareto sets of favourable designs as shown above.
Pant, S., Limbert, G., Curzen, N. and Bressloff, N. W. , 2011, Multi-objective design optimisation of coronary stents, Biomaterials, to appear as a Leading Opinion Paper. PDF.
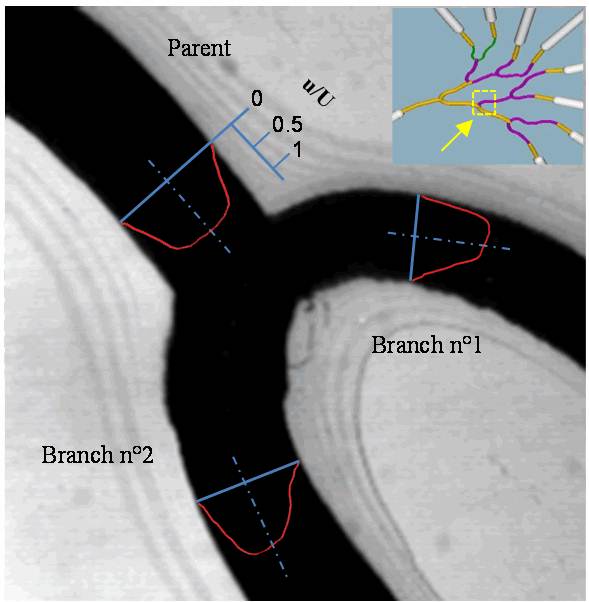
Flow disturbance.Click on the images below to compare the flow in and around the struts of different stent designs.
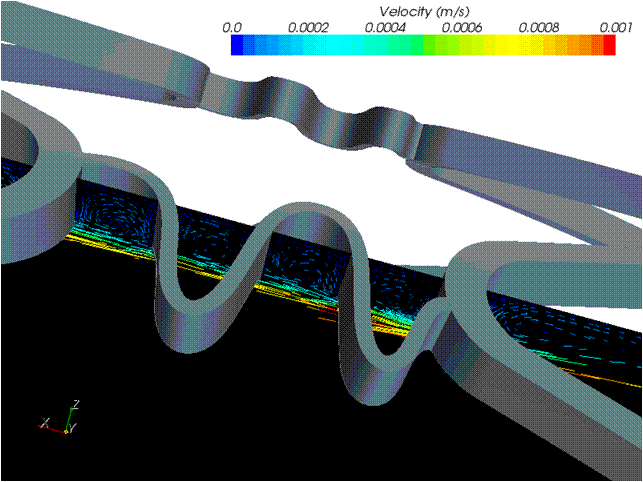
See: Bhaskar, K. V., Bressloff, N. W., Nair, P. B. and Shearman, C. P., 2008, Predictive haemodynamics in a one-dimensional human carotid artery bifurcation. Part II: Application to graft design, IEEE Trans. Biom. Eng., 55(3): 1176-1184. PDF
Hip replacement. Researcher: Hamidreza Alidousti. Other academic: Mark Taylor.
The stem of cementless total hip implants is only partially in contact with the surrounding bone leaving periprosthetic gaps around the implant, which may be in communication with the joint capsule. It has been suggested that periprosthetic fluid flow and high pressure may play an important role in osteolysis generation in the proximity gaps. To explain this phenomenon, the concepts of 'effective joint space' and 'pumping stem' have been cited in may studies. However, there is no clear understanding of the factors causing, or contributing to, these mechanisms. In order to obtain a better understanding of the main contributors to periprosthetic flows and their involvement in osteolysis generation, steady state and transient 2D computational models of the lateral side of a stem-femur system including the joint capsule, an open gap to the capsule and the surrounding bone are studied. It is hypothesised that capsular pressure, gap dimensions and micromotion of the gap during cyclic loadings of an implant can play a defining role in inducing periprosthetic flow.
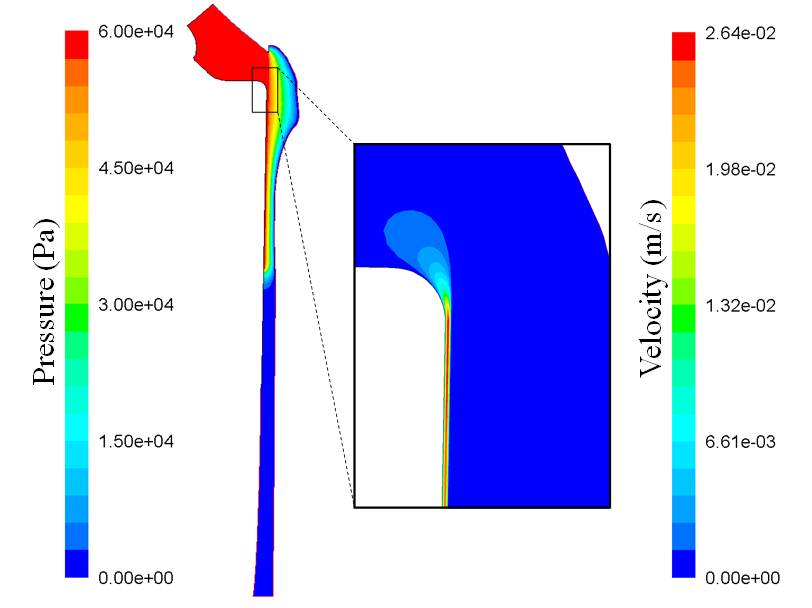
It is shown that high capsular pressure may be the main driving force for high fluid pressure and flow in the bone surrounding the gap, while micromotion of only very long and narrow gaps can cause significant pressure and flow in the bone. At low capsular pressure, micromotion induces large flows in the gap region; however, the flow in the bone tissue is almost unaffected. The results also suggest the existence of high velocity spikes in the bone region at the bottom of the gap. High capsular pressure is observed to be the main cause of these velocity spikes whereas, at low capsular pressure, gap micromotion of only very long and narrow gaps generates significant velocity spikes in the bone at the bottom of the gaps
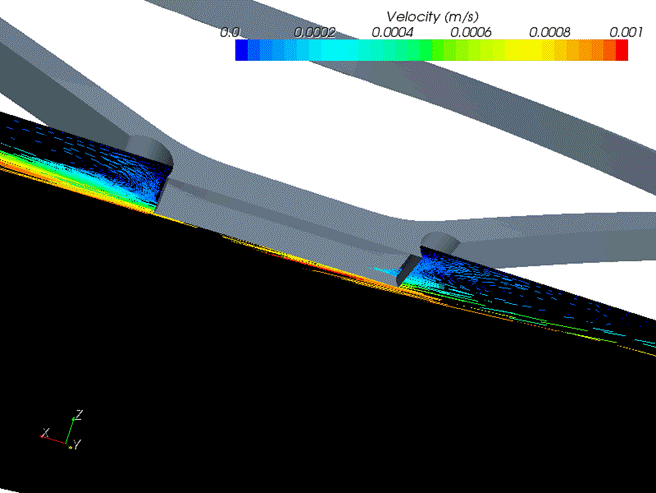
Lab-on-a-chip systems. Researcher: Dario Carugo. Other academic: Xunli Zhang.Due to the complexity and limitations associated with in-vivo analysis, the need grows for compact in-vitro models to be used in microcirculation reaserch in order to mimic the geometry of the microvascular network. A microfluidic-based model of the microcirculatory system was designed and fabricated using a micromilling technique followed by bonding of the two polymethyl methacrylate (PMMA) sheets with milled microchannels. The model geometry was reproduced from representative computational models of vascular architecture.
Very close agreement between experiment and computation has been obtained for the cell depletion layer thickness.

