| Solidification of steels | page 1 of 8 | |
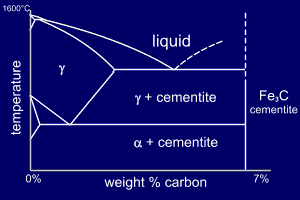 |
In their simplest form,
steels are alloys of Iron (Fe) and Carbon (C). The Fe-C
phase diagram is shown below, up to around 7%
Carbon. This is a fairly complex phase
diagram but, as we are only interested in the steels part
of the diagram we can make a few simplifications. Steels have been so important to engineers for so many years that each phase has inherited a name as well as a Greek letter. The phase diagrams for steels are on p24 of your book. |
| Solidification of steels | page 2 of 8 | |
 |
The gamma phase
is called austenite.
Austenite is a high temperature phase and has a Face Centred
Cubic (FCC) structure [which is a close packed
structure]. The alpha phase is called ferrite. Ferrite is a common constituent in steels and has a Body Centred Cubic (BCC) structure [which is less densely packed than FCC]. Fe3C is called cementite and lastly (for us), the "eutectic like" mixture of alpha+cementite is called pearlite. |
| Solidification of steels | page 3 of 8 | |
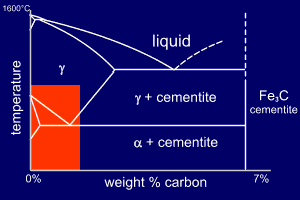 |
The problem can be
simplified by accounting for the following two points :
|
| Solidification of steels | page 4 of 8 | |
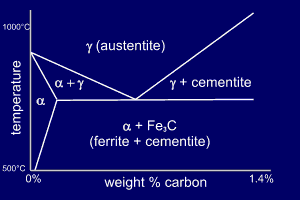 |
The phase diagram shows the
Fe-C phase diagram up to around 1.4%C
and 1000ºC. This appears to
cause a problem - there is no liquid phase but otherwise,
in form, the phase diagram looks like our
"standard" phase diagram. In fact, although the
reactions occur in the solid state they can be treated in
exactly the same way as if they included the liquid
state. There is, though, one proviso. The word eutectic is replaced by the word eutectoid (eutectic-like) to show that the reaction is in the solid state. . |
| Solidification of steels | page 5 of 8 | |
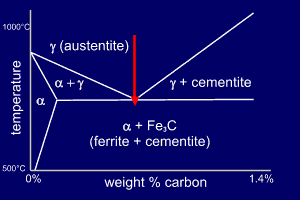 |
The eutectoid composition is
Fe-0.83wt%C and at this
composition the high-temperature austenite will undergo
the eutectoid reaction at 723ºC:
The ferrite and cementite grow co-operatively as a lamellar mixture (pearlite). |
| Solidification of steels | page 6 of 8 | |
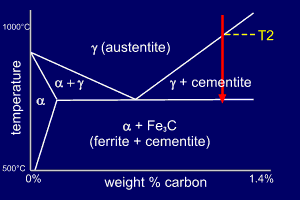 |
An alloy of composition Fe-1.3wt%C
is to the right of the eutectoid point and
so it is termed hypereutectoid steel. As the austenite crosses the phase line at T2 some of the austenite will transform into cementite and so the remaining austenite will become richer in iron. Energetic considerations show that the cementite will (and does) form at the austenite grain boundaries. When the steel reaches the eutectoid temperature the remaining austenite will be of eutectoid composition and transforms into pearlite (alpha+cementite). So, the final microstructure will contain cementite at the grain boundaries (pro-eutectoid cementite) and pearlite (eutectoid). |
| Solidification of steels | page 7 of 8 | |
|
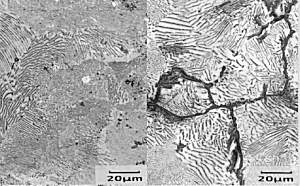
Samples of hypereutectoid
steel normally show less pro-eutectoid
cementite at the edge than in the rest of
the sample. This is due to decarburisation
in the surface layers at high temperatures (carbon
diffuses out of the surface of the sample). Although this is an unwanted effect the reverse effect is commonly used. A component is placed in a hot carbon-rich environment which encourages diffusion of carbon into the surface of the steel, increasing the surface hardness. This is called case carburising. |
| Solidification of steels | page 8 of 8 | |
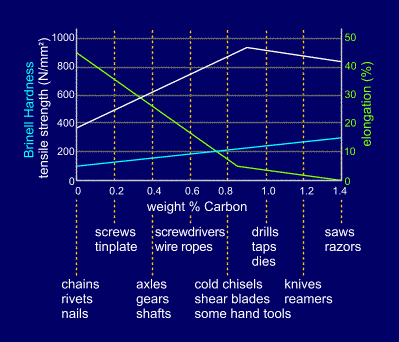 |
The microstructures of
steels vary considerably with carbon content, with
increasing amounts of the hard, brittle, cementite
being present in steels of higher carbon content. This
variation in microstructure leads to significant changes
in engineering properties, as shown in the figure. For example, strength increases with carbon content up to the eutectoid composition but then starts to drop as a grain-boundary network of brittle cementite is formed.
|
| Solidification of steels | more info | |
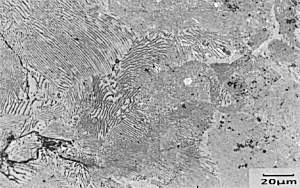 |
Micrograph of
Fe-0.83wt%C The specimen has been metallographically prepared and etched in boiling alkaline sodium picrate (very dangerous and explosive!) which stains the surface of the cementite brown/black and leaves the ferrite unattacked. |
| Solidification of steels | more info | |
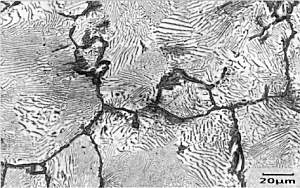 |
Micrograph of
Fe-1.3wt%C The specimen has been metallographically prepared and etched in boiling alkaline sodium picrate (very dangerous and explosive!) which stains the surface of the cementite brown/black and leaves the ferrite unattacked. |