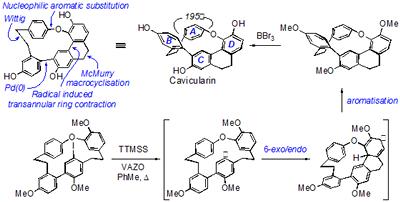
Research project: Harrowven: Total Synthesis of Cavicularin and Riccardin C
Cavicularin, from the liverwort Cavicularia densa, is one of the most unusual and synthetically challenging natural products to have been isolated in the last decade. Comprised of a macrocyclic core containing dibenzyl and dihydrophenanthrene units conjoined by a biaryl bond and an ether linkage, this imparts such strain on the system that arene A is forced to adopt a boat like configuration, being twisted out of the plane by some 15° in the solid state (see Figure).