| Solidification of Al-Si alloys | page 1 of 6 | |
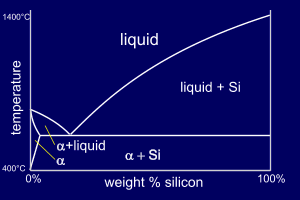 |
Al-Si alloys differ from our
"standard" phase diagram in that aluminium has zero solid
solubility in silicon at any temperature. This means
that there is no beta phase and
so this phase is "replaced" by pure silicon
(you can think of it as a beta phase which consists only
of silicon). So, for Al-Si alloys, the eutectic composition is a structure of alpha+Si rather than alpha+beta. You can find this phase diagram on p22 of your book. |
| Solidification of Al-Si alloys | page 2 of 6 | |
 |
Consider two Al-Si alloys,
both of composition 13wt%Si. The second alloy, though,
will be "doped" with a very small amount of
Sodium (0.01%Na). So, the two alloys are :
13wt%Si places the alloy to the right of the eutectic point on the phase diagram and so the Al-13wt%Si alloy is hypereutectic. |
| Solidification of Al-Si alloys | page 3 of 6 | |
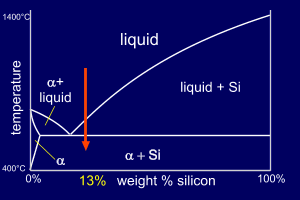 |
The eutectic on this phase
diagram contains much more alpha
than Si and so we expect the
eutectic mixture (alpha+Si) to be mainly alpha (consider
the lever rule at the eutectic temperature). As this alloy is hypereutectic, primary Si forms first, depleting the liquid of Si until it reaches the eutectic composition where the remaining solidification follows the eutectic reaction. |
| Solidification of Al-Si alloys | page 4 of 6 | |
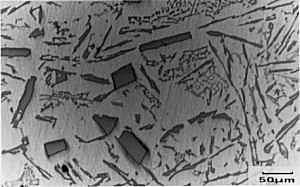 |
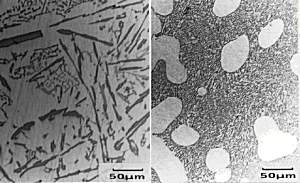
The primary Si has a
cuboidal form which can be seen in the micrograph. The
eutectic mixture, though, is non-lamellar in form and
appears, in section, to consist of separate flakes
(although studies have shown that the flakes are, in
fact, interconnected three dimensionally). So, why do we care? Well, these coarse flakes of Si in the eutectic promote brittleness within these alloys. Most Al-Si alloys used have a near-eutectic composition since this gives a lower melting point and makes them cheaper to cast. If these alloys are to be of any great use, we must improve their properties somehow and deal with the brittle Si flakes. The sample has been metallographically prepared (mounted, ground and polished). No etching is required as Si appears grey whilst the alpha phase appears white. |
| Solidification of Al-Si alloys | page 5 of 6 | |
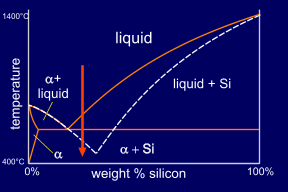 |
Adding a small quantity of a ternary element, here Sodium, causes modification of the microstructure. This addition effectively moves the eutectic point to a higher silicon concentration and lower temperature. This modifies the growth of the eutectic silicon to produce an irregular fibrous form rather than the usual flakes. The eutectic point has moved far enough to make the alloy, at this composition, hypo-eutectic instead of hyper-eutectic. So now primary alpha forms, rather than primary Si. This can be seen on its micrograph. |
| Solidification of Al-Si alloys | page 6 of 6 | |
|
|
| Solidification of Al-Si alloys | more info | |
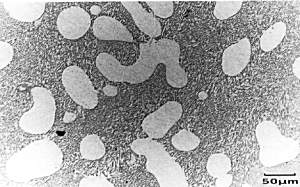 |
Micrograph of
Al-13%wtSi-0.01%Na alloy White blobs of primary alpha dendrites can be seen, surrounded by a fine, fibrous eutectic mixture of alpha and Si. |