| Eutectic alloys | page 1 of 5 | |
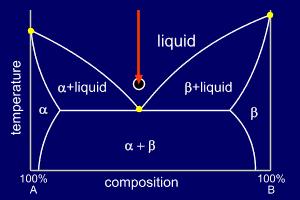  |
Consider a mixture of
elements A and B in their eutectic proportions at a high
enough temperature to make the mixture fully liquid. Each diagram is marked with an arrow at the eutectic composition and indicating the current temperature (the start temperature in this case). The circle below the diagram shows a stylised representation of the alloy's microstructure (here showing nothing of interest as the alloy is liquid). |
| Eutectic alloys | page 2 of 5 | |
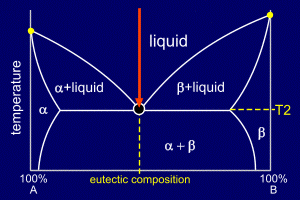  |
The mixture is slow
cooled, undergoing no change in state until
it reaches temperature T2,
where it starts to solidify at any favourable
nucleation sites. Note that the alloy forms into "stripes" which are alternate layers of alpha and beta. These layers are often only of the order of 1 micron across and the reason that the eutectic forms in this way can be understood by considering the diffusion times required to form the solid. The "stripy" microstructure is known as lamellar microstructure. |
| Eutectic alloys | page 3 of 5 | |
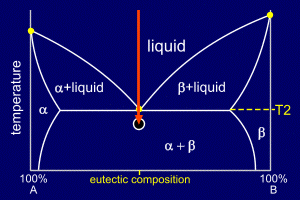  |
As the alloy continues to
cool the existing nucleation sites will grow, adding alpha
to alpha and beta to beta. These nucleating and growing regions of solid alloy will form grains. Grain boundaries occur where growing grains, which will be of differing orientations and from different nucleation sites, meet. Further nucleation sites will continue to form within the liquid parts of the mixture. Remember, though, this happens over a very short time scale and with no further decrease in temperature. The eutectic composition solidifies, like a pure element, at a single temperature, not over a temperature range. |
| Eutectic alloys | page 4 of 5 | |
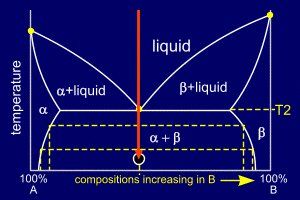  |
The entire mixture rapidly
solidifies into a eutectic solid. Looking at the phase diagram, it can be seen that the compositions of alpha and beta change with temperature. By considering tie lines and the phase diagram, it can be seen that beta has a decreasing proportion of A in it as the temperature decreases. Similarly the proportion of B in alpha decreases. This means that even though the alloy is now solid, the composition of the stripes of alpha and beta must continue to change as it cools (to room temperature for example). Atoms of A and B will diffuse across these "stripes" to produce the equilibrium compositions of alpha and beta at a given temperature. |
| Eutectic alloys | page 5 of 5 | |
  |
|