| Hypo-eutectic alloys | page 1 of 6 | |
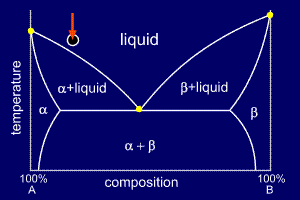  |
Now, consider a mixture of A
and B so that the overall composition of the alloy places
it to the left of the eutectic
point. Initially the alloy is at a high enough
temperature to ensure that the mixture is fully liquid. The diagram is marked with an arrow at the correct composition and indicates the current temperature (the start temperature in this case). The circle below gives a stylised representation of the alloy's microstructure (here showing nothing of interest as the alloy is liquid). When the composition of an alloy places it to the left of the eutectic point it is called hypo-eutectic. Note, though, that this is merely convention. If the phase diagram had been drawn the other way around, with 100%A on the right and 100%B on the left, then the same alloy would be called hyper-eutectic, as it would be to the right of the eutectic point. |
| Hypo-eutectic alloys | page 2 of 6 | |
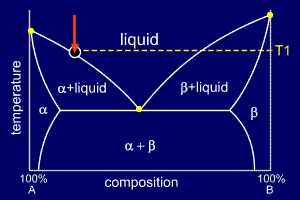  |
The mixture is slow cooled,
undergoing no change in state until it reaches
temperature T1, when it reaches
the liquidus line. Here, as the labelling suggests, alpha
starts to solidify at any favourable
nucleation sites. The alpha solidifies as dendrites which grow to become grains of alpha. The first solid to form is called the primary solid and so, in this case, primary alpha is formed. |
| Hypo-eutectic alloys | page 3 of 6 | |
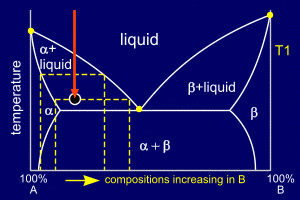  |
As the alloy continues to
cool the existing nucleation sites will grow and further
nucleation sites will continue to form within the liquid
parts of the mixture. These nucleating and growing regions of solid alloy form grains and when these meet grain boundaries are formed. The primary alpha dendrites grow, which accounts for the shapes the alpha forms in cross sectional samples. As the remaining liquid cools its composition becomes richer in B (effectively sliding down the liquidus line). The composition of the solid alpha also becomes richer in B, as shown by the phase diagram. If cooling is not sufficiently slow (i.e. non-equilibrium) then B atoms will not be able to diffuse into the centres of the grains of alpha. If this is the case then coring will occur. |
| Hypo-eutectic alloys | page 4 of 6 | |
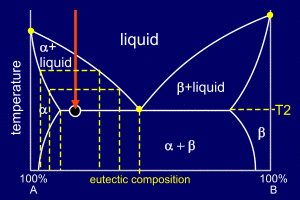 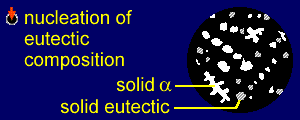 |
As alpha solidifies it
removes A and B atoms from the remaining liquid. Alpha is
mostly A (with a small amount of B) and so the remaining
liquid becomes relatively richer in B.
This continues until enough A has been removed so that
the remaining liquid - sliding down the liquidus line -
is of eutectic composition. This composition will be achieved at temperature T2, at the point where the temperature crosses the eutectic line. At this point alpha stops forming as a discrete solid and the remaining liquid starts to solidify into the stripy (lamellar) eutectic composition of alpha and beta. Therefore, solid eutectic forms. |
| Hypo-eutectic alloys | page 5 of 6 | |
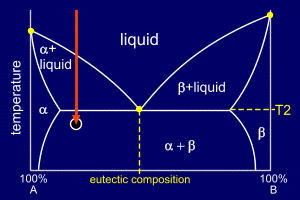 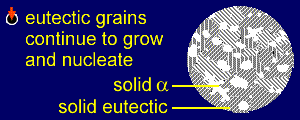 |
The existing eutectic
nucleation sites will grow, adding alpha to the stripes
of alpha and beta to the stripes of beta in the eutectic
regions. New sites will continue to form. Note that, unlike the alpha solidification, it is not necessary to continue decreasing the temperature to achieve full solidification. The eutectic liquid solidifies in the same way as a pure solid, at a specific temperature. |
| Hypo-eutectic alloys | page 6 of 6 | |
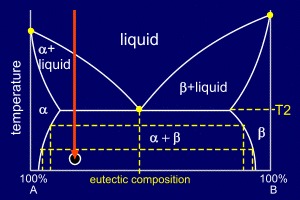 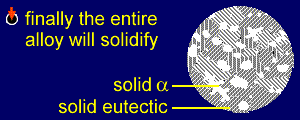 |
The entire alloy has now
solidified into a mixture comprising grains
of alpha and grains of eutectic
mixture (alpha and beta). The diffusion processes through the solid, which occur as the alloy cools, are more complex than those of the eutectic case. As with a eutectic alloy, the amount of B in the alpha phase changes with temperature, and so B will have to diffuse through the alpha. This diffusion must also occur in the grains of pure alpha, as the composition of alpha also changes. This can be seen by examining the diagram.
|