| Solidification of Al-Cu alloys | page 1 of 5 | |
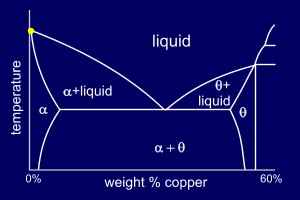 |
Observant people may have
noticed that the Al-Cu phase
diagram shown only goes up to around 60%, by weight, of
Copper. The Al-Cu phase diagram is split at around 54wt%Cu
by a particular phase. This "split" means that
the two parts of the diagram can be considered
separately. The diagram up to the 54% point is very
similar to the "standard" phase diagram. When a phase diagram, such as this one, has an intermetallic phase it is not named alpha or beta but is assigned another Greek letter. Unfortunately there is no system to this naming, it is purely convention. Here the "right" hand phase is named theta but other than its name it is dealt with in exactly the same way as a beta phase. This diagram is on p20 in your books. |
| Solidification of Al-Cu alloys | page 2 of 5 | |
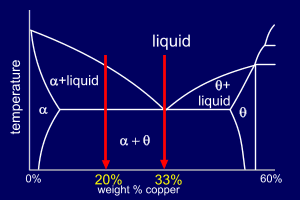 |
Consider two Al-Cu alloys,
one of composition 33wt%Cu and
the other of 20wt%Cu. Examining the phase diagram:
So, first considering the Al-33%Cu alloy: |
| Solidification of Al-Cu alloys | page 3 of 5 | |
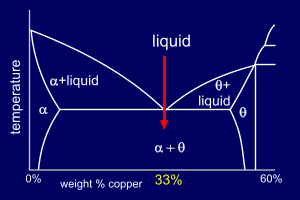 |
The eutectic reaction, at 33%Cu
can be shown as:
So, the two phases grow simultaneously as an interconnected structure - the eutectic phase. The lamellar nature of the eutectic mixture as it solidifies ensures that diffusion fields ahead of the liquid-solid interface are limited (i.e. the atoms do not have to travel vast distances for the two phases to form simultaneously) |
| Solidification of Al-Cu alloys | page 4 of 5 | |
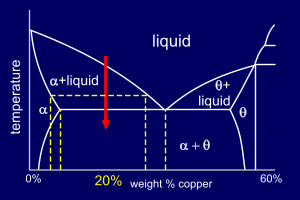 |
Now, the Al-20wt%Cu
alloy is hypoeutectic.
The liquid alloy initially contains a higher percentage
of Al than that corresponding to the eutectic
composition; the primary solid
phase that forms is therefore alpha. Formation of the alpha phase, however, depletes the remaining liquid in Aluminium (Al) and the liquid composition shifts toward the eutectic composition. When the composition reaches eutectic (33%Cu for this phase diagram) the remaining liquid follows the eutectic reaction. So, at room temperature, the microstructure consists of primary alpha dendrites surrounded by a finely divided eutectic mixture of two solid phases (alpha+theta). |
| Solidification of Al-Cu alloys | page 5 of 5 | |
 |
Now, the question presents
itself : "Why do I want to know this ?" Well, microstructural changes strongly influence the engineering properties of an alloy. The lamellar nature of the eutectic phase, for instance, impedes dislocation movement through the alloy. If dislocations cannot move easily then the material won't yield until a higher stress is applied, i.e. the strength of the alloy is increased.
|
| Solidification of Al-Cu alloys | more info | |
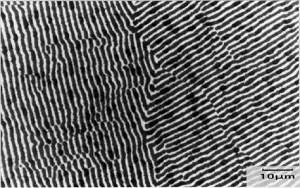 |
Micrograph of
Al-33%wtCu alloy This specimen has been metallographically prepared (mounted, ground and polished) and then etched in dilute (10%) nitric acid which stains the surface of the theta phase brown/black whilst leaving the alpha phase unattacked - thus appearing white. The specimen was then photographed under a microscope. The scale is shown in the bottom corner of the micrograph. |
| Solidification of Al-Cu alloys | more info | |
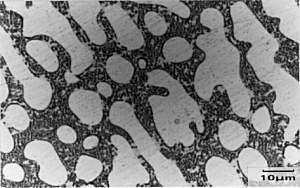 |
Micrograph of
Al-20%wtCu alloy This specimen has been metallographically prepared (mounted, ground and polished) and then etched in dilute (10%) nitric acid which stains the surface of the theta phase brown/black whilst leaving the alpha phase unattacked - thus appearing white. The specimen was then photographed under a microscope. The scale is shown in the bottom corner of the micrograph. |