Could immunotherapy help patients with a rare and often incurable cancer?
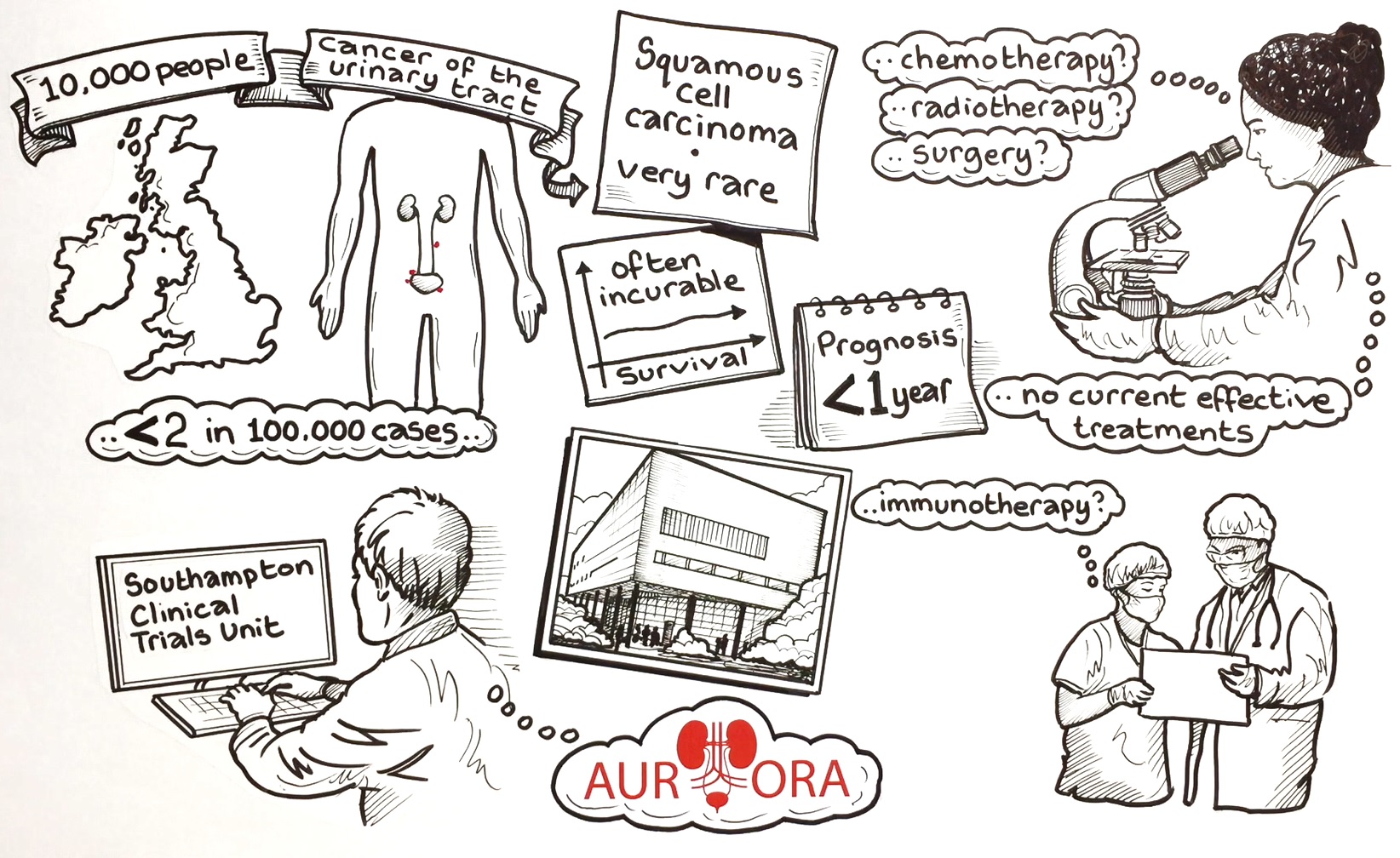
A new clinical trial that will look at whether an immunotherapy treatment could improve outcomes for patients with a rare and often incurable form of cancer, has opened to patients.
The AURORA trial, which is funded by Cancer Research UK and is being run by the Cancer Research UK Southampton Clinical Trials Unit, is now recruiting at University Hospital Southampton, with more sites soon to be opened.
Urinary tract squamous cell carcinoma (UTSCC) is a rare form of cancer of the urinary tract including the bladder. At present there are no treatment options that are proven to extend survival in this disease. This is, in part, because of research having been limited in this disease in the past. Typically, survival rates are poor, and prognosis is under a year.
Immunotherapy works by helping your body’s own immune system to recognise and destroy cancer cells. This approach to treatment has already become established in a number of other cancers.
The AURORA trial will study whether an immunotherapy called atezolizumab, which blocks a protein that stops immune cells from working properly to target some cancers, can be effective for patients with urinary tract squamous cell carcinoma (UTSCC).
Dr Simon Crabb, Associate Professor of Medical Oncology and Chief Investigator of the AURORA trial, says:
“Because UTSCC is such a rare cancer, there are very few clinical trials conducted into the disease and therefore no current effective treatments, meaning the disease is often incurable. We hope that this trial will show whether immunotherapy could provide a treatment option for patients in the future.”
Professor Gareth Griffiths, Director of the Cancer Research UK Southampton Clinical Trials Unit and co-chair of the International Rare Cancers Initiative (IRCI) Rare Geniturinary Cancer Working Group said: “It is fantastic that we are opening a trial in a rare cancer type where there have been a limited number of clinical trials to date for our cancer patients. AURORA will be conducted in the UK but if we see evidence the treatment is safe and effective, we then plan to develop future larger trials internationally across our IRCI partners”.
AURORA has received over £814,000 from Cancer Research UK, with further support from pharmaceutical company Roche who are providing the atezolizumab for the trial.