
Patients
Information for study participants and patients interested in taking part in the BRITISH study.


Information for study participants and patients interested in taking part in the BRITISH study.

Information and resources for research sites who are running the BRITISH study.
Background and study aim
Non-ischaemic cardiomyopathy (NICM) is a common cause of heart failure accounting for around a third of cases in the UK. 'Non-ischaemic' refers to the fact that the weakness of the heart muscle that characterises the condition is not primarily due to the blood flow down the coronary arteries.
Some patients with NICM have a higher risk of experiencing serious abnormal heart rhythms that might be life threatening. Current guidelines recommend doctors consider fitting a device called an Implantable Cardioverter-Defibrillator (ICD) that can correct these serious heart rhythms by delivering an electric shock to the patient’s heart.
The current guidelines look at how well the heart is pumping to decide which patients should get an implant. However, we know from previous research studies as many as 90% of patients who have an ICD will never need to use it because they won’t experience any serious heart rhythms.
Research has shown that patients who have scar tissue in their heart, seen on Cardiac Magnetic Resonance Imaging (CMR), are at a higher risk of dying suddenly from an abnormal heart rhythm.
The BRITISH study will test whether the presence of scar tissue on the heart scan (CMR) can be used to decide if patients need an ICD or not.
Who can participate?
Adults over the age of 18 years with Non-Ischaemic Cardiomyopathy who have severe heart failure despite 3 months of optimum medical therapy and have the presence of scar tissue on their routine heart scan (CMR).
What does the study involve?
Participants will be randomly assigned (like the toss of a coin) to one of two study arms. Half the study participants will receive an ICD and the other half to no ICD. For participants that are allocated to no ICD, we’d still like to monitor the heart rhythm and this can be done simply by fitting a device called an Implantable Loop Recorder (ILR) which records the heart rhythm.
Both groups will be followed up to decide whether having an ICD fitted reduces the chances of dying from an abnormal heart rhythm.
What are the possible benefits and risks of participating?
There may be no personal benefit for participants in this study. We hope there may be some benefit to patients in not having an ICD fitted if they don’t need one. However, we can’t be sure of this and this is why we’re doing the study.
Even if there is no personal benefit to participants, we hope this study will increase our knowledge and improve how we manage patients with this condition in the future. We hope the study results might lead to a change in the guidelines used by doctors when they decide which heart failure patients should have an ICD fitted.
Whether patients decide to take part in the research study or not, there are certain risks associated in having an ICD fitted, and these are outlined in the Participant Information Sheet. The study will not increase or decrease any risk around this procedure.
The BRITISH study is funded by a research grant from the British Heart Foundation and is sponsored by University Hopsitals Southampton NHS Foundation Trust.


This page provides information for study participants and patients interested in finding out more or joining the BRITISH study. Information for researchers and site teams can be found her e .
Find out more about what taking part in the BRITISH study involves - watch the BRITISH video on Explain My Procedure .
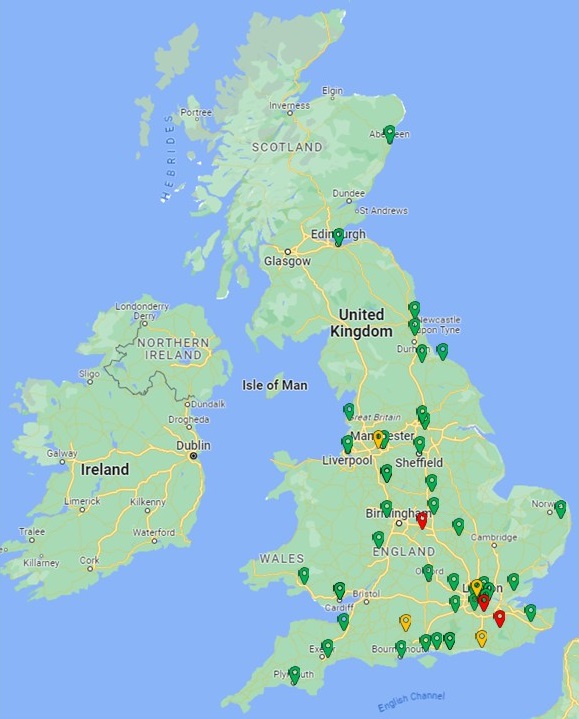
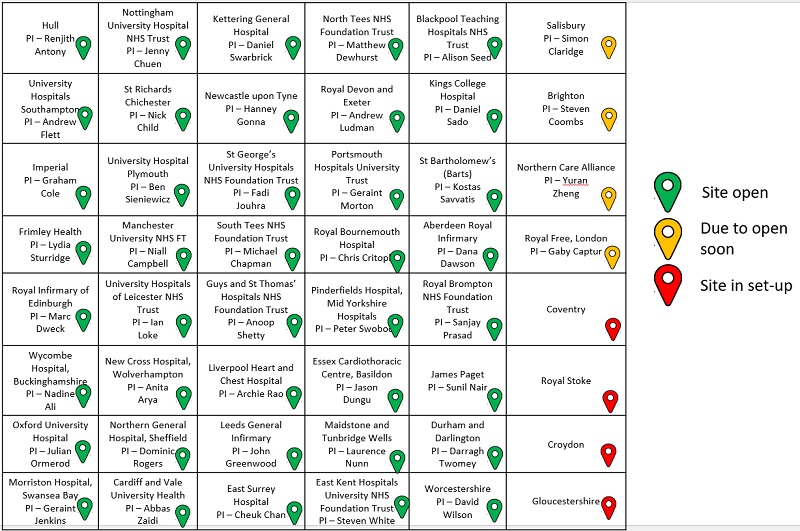
If you would like to take part in the BRITISH study you should speak to your cardiologist to find out if you are eligible for recruitment and whether the study is open near you. Please be aware that if you already have ICD fitted you are not eligible for this study.
Click here to download the full Participant Information Sheet
Coming soon
This page provides information for researchers and research teams at sites taking part in the BRITISH study. Information for study participants or a patients wanting to find out more information about joining the study can be found here .

Downloadable trial documentation for sites running the BRITISH study.

Videos and documents to help sites in the set-up and running of the BRITISH study.
Coming soon.
For sites needing to contact the MRI Corelab with queries regarding patients MRI scans or scan uploads, please email british@mycardium.com .
The imaging manual will be uploaded here soon.


The first patient has undergone surgery in a landmark clinical trial which aims to reduce deaths from sudden cardiac arrests.

The BRITISH trial, a landmark clinical trial which aims to reduce deaths from sudden cardiac arrests, opens to the first patients in Southampton.

A team of Southampton researchers have been awarded £1.8m from the British Heart Foundation to run a new clinical trial aimed at improving treatment for people diagnosed with heart failure.