Trial Overview
Trial Team
Essential Trial Documention
MANCAN2 Exercises
Title
MANCAN2 (MANaging symptoms during prostate CANcer treatment): A multicentre randomised controlled trial (RCT) of a virtual self-help cognitive behavioural therapy (CBT) intervention to reduce the impact of hot flush and night sweat (HFNS) symptoms in men with prostate cancer undergoing androgen deprivation therapy (ADT).
Description
Hormone therapy medication is widely used to treat prostate cancer. It works by blocking or reducing the amount of testosterone in the body. This stops or slows down the cancer's spread. While effective as a medication, 80% of men suffer from Hot Flushes and Night Sweats as a result of taking it. These side effects can occasionally be so troublesome that some men decide to stop taking the medication allowing the cancer to grow.
Unfortunately, there are few established effective treatments for men with Hot Flushes and Night Sweats. Our previous research has shown that cognitive behavioural therapy (CBT), a type of talking therapy, can help both men and women manage hot flush and night sweats in a positive way by changing how they think and behave.
This study will evaluate how effective a self-help CBT programme is in reducing the impact of hot flushes and night sweats in men taking hormone therapy for prostate cancer. This will be delivered by a trained member of the existing prostate cancer nursing team.
We will recruit 150 men with a diagnosis of prostate cancer currently receiving hormone therapy and experiencing problematic hot flushes and night sweats from seven hospitals around the UK. Half will be randomly assigned to a 4- week self-help CBT programme and the others will receive treatment as usual.
Participants randomised to the self-help programme will be given a cognitive behavioural therapy booklet to work through consisting of information and exercises to help manage symptoms and improve wellbeing. There will also be two virtual group workshops delivered by the cancer nurse specialist team. Men in this arm will also receive a CD/ audio file demonstrating breathing and relaxation exercises.
All participants will complete study questionnaires at baseline, 6 weeks, and 6 months.
Objectives
Primary:
- To determine whether the addition (to Treatment As Usual (TAU) of virtual self-help Cognitive Behavioural Therapy (CBT), delivered by a patient’s existing prostate cancer Clinical Nurse Specialist (CNS) team, reduces the impact of HFNS at 6 months post randomisation in men with prostate cancer undergoing ADT
Secondary:
To determine:
- The effect of the intervention on the impact of HFNS at 6 weeks post randomisation
- The effect of the intervention on HFNS frequency
- The effect of the intervention on men’s HFNS beliefs and behaviours
- The effect of the intervention on quality of life (QoL)
- The effect of the intervention on other symptoms including anxiety, depression, mood and sleep
- The effect of the intervention on men’s compliance with ADT
- The level of fidelity of the CBT when delivered by a patient’s existing prostate cancer clinical nurse specialist (CNS) team
- Resource use analyses
- Prostate cancer CNS team experiences of introducing this new intervention
- Participant acceptability of the intervention
- Explore barriers and facilitators to implementing the intervention into routine practice
- Health economics of the intervention
Trial Design
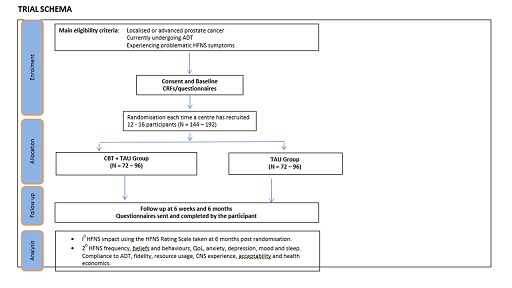
A multicentre randomised controlled trial of the addition of a virtual self-help CBT intervention to TAU versus TAU alone.
Trial Status
In set-up
Population
MANCAN2 will recruit approximately 150 (144-192) men with prostate cancer currently receiving ADT and experiencing problematic HFNS symptoms (defined as a HFNS rating scale score of two or more). We will include patients receiving ADT in both the localised (curative) and advanced (palliative) settings with stratification for disease status. To minimise risk that patients might be symptomatic from their cancer, such that data integrity and outcomes might be influenced, we will recruit advanced disease patients only with hormone sensitive metastatic prostate cancer (excluding later stage castrate resistant disease).
.jpg)
This trial is funded by a Research for Patient Benefit (RfPB) grant from the NIHR (award reference no. NIHR201542)
Senior Trial Manager:
Zina Eminton
Trial Manager:
Alannah Morgan
Trial Coordinator:
Alice Leaper
Contact Information for trial queries:
Email: mancan2@soton.ac.uk
Phone: 023 8120 5589
SAE Reporting
Email: ctu@soton.ac.uk
Essential Documents
MANCAN2 - Protocol_v1 02-Oct-2021
MANCAN2 - Patient Information Sheet_v2 08-Dec-2021
MANCAN2 - Participant Information Sheet_CNS Team Member_ v1 02-Oct-2021
MANCAN2 - Participant Information Sheet_Medic_ v1 02-Oct-2021
MANCAN2 - Participant Information Sheet_Manager_ v1 02-Oct-2021
MANCAN2 - Patient Informed Consent Form_v2 08-Dec-2021
MANCAN2 - Informed Consent Form_CNS Manager Medic_ v1 02-Oct-2021
MANCAN2 - Site Contacts Form_ v1 02-Oct-2021
MANCAN2 - Eligibility Checklist_ v1 02-Oct-2021
MANCAN2 - Invite Pack_v2 08-Dec-2021
MANCAN2 - Patient Trial Card_v1 01-Nov-2021
MANCAN2 - Baseline Form_v2 20-Jan-2022
MANCAN2 - End of Study Form_ v1 02-Oct-2021
MANCAN2 - Patient Evaluation Questionnaire_Site Instructions_ v1 02-Oct-2021
MANCAN2 - Patient Evaluation Questionnaire_v1 02-Oct-2021
MANCAN2 - Resource Useage Questionnaire_v1 02-Oct-2021
MANCAN2 - NoMAD Questionnaire_SCTU Instructions_ v1 02-Oct-2021
ISF Documents
MANCAN2 - Study Contacts and Information Page_v1 06-Jan-2022
MANCAN2 - Master Patient List_v1 02-Feb-2022
MANCAN2 - Screening and Recruitment Log_v1 01-Feb-2022
MANCAN2 - Site Delegation Log_v1 20-Oct-2021
MANCAN2 - Site Visit Log_v1 31-Jan-2022
