Title
SynthEtic LEthal Cancer Therapy in mesothelioma (SELECTmeso): a molecularly stratified multi-arm phase II platform trial for patients with relapsed malignant mesothelioma
Description
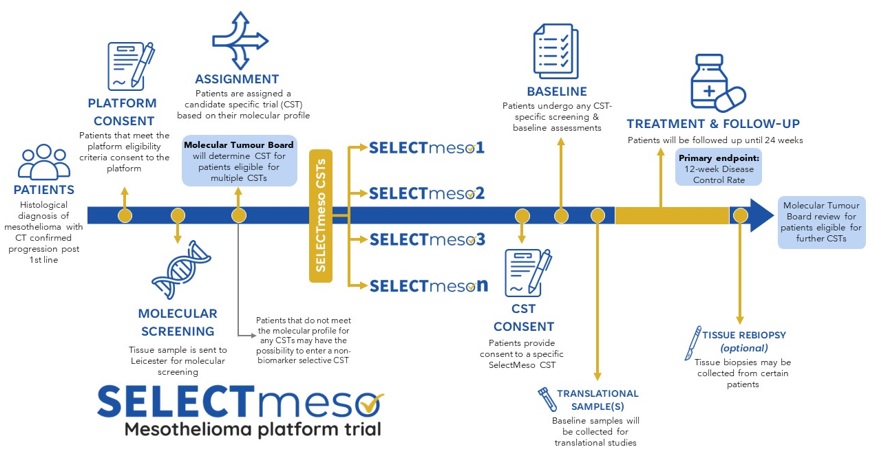
SELECTmeso is a UK, multicentre, multi-arm, adaptive, phase II platform trial to determine the activity and safety of multiple targeted therapies for the treatment of patients with relapsed malignant mesothelioma in molecularly stratified cohorts. SELECTmeso builds on the 5-arm MIST umbrella platform which was the first ever pilot for precision therapy for mesothelioma.
A key feature of SELECTmeso is the integration of deep multi-omic profiling (exomic, transcriptomic and ultra-deep spatial phenotyping) to interrogate features that correlate with extremes of drug sensitivity (1, 2).
This platform allows for the assessment of multiple precision therapies, with the ability to add drug candidates based on an underlying scientific hypothesis and rationale. Promising candidates may move to an external randomised trial for further evaluation outside of SELECTmeso.
Objectives
Primary:
- To determine the molecular subtype of mesothelioma to determine eligibility for SELECTmeso trial candidates which are open for recruitment.
Secondary:
- To conduct in-depth molecular profiling of tumour blocks to enable exploration of the molecular determinants of sensitivity and acquired drug resistance. Register with National Cancer Registration and Analysis Service to obtain long-term clinical outcomes after completion of SELECTmeso candidate specific trials such as disease progression and overall survival.
CST (Candidate Specific Trial) Primary:
- To determine the Disease Control Rate (DCR) at 12 weeks.
CST (Candidate Specific Trial) Secondary:
- To determine DCR at 24 weeks, response rate, progression-free and overall survival (up to 24 weeks), safety and tolerability.
Trial Design
Each CST will use a Bayesian Optimal Phase II (BOP2) design which incorporates at least one ‘go/no-go’ interim analysis, this minimises the projected sample size if a low number of patients displaying disease control rate (DCR) is observed. Once each trial arm reaches the required sample size, we can conclude whether the treatment is acceptable, in terms of activity at a pre-determined DCR rate, safety and feasibility of use, and so warrants further evaluation in a future randomised trial.
Based on a one-sided type I error rate of no more than 0.05, a sample size of 26 provides power of approximately 0.83 under the Bayesian Optimal Phase 2 (BOP2) design. This considers an interim analysis for futility (not pausing recruitment) after 13 patients, and a null hypothesis that the 12-week DCR is 0.25 (i.e., a true DCR of 25% at 12 weeks would be too low, requiring no further evaluation) against an alternative hypothesis that the DCR is 0.5 (i.e., a true DCR of 50% at 12 weeks would be sufficient to warrant further evaluation).
The BOP2 design recommends stopping for futility if 3 or fewer patients out of 13 achieve disease control. The numbers for the interim analysis are to provide guidance to the Trial Management Group and may be overruled if there are other possible reasons (e.g., toxicity) that outweigh the recommendation to stop or continue.
The primary endpoint is the proportion of patients who have achieved disease control (complete response, partial response, or stable disease) assessed by mRECIST 1.1 at 12 weeks. A total of 11 patients achieving disease control or more in the 26 patients will suggest that the treatment is sufficiently effective.
Trial Status
In set-up
Population
Patients with confirmed histological diagnosis of mesothelioma (any histology) with evidence of MTAP loss and wild-type P53 on immunohistochemistry, and evidence of disease progression following prior standard systemic therapy on CT scan.
Inclusion:
- Histological confirmation of malignant mesothelioma (pleural, or non-pleural)
- Evidence of disease progression on CT scan
- Available archival tissue block for molecular screening
- Previous treatment with at least 1st line licenced systemic anti-cancer therapy. Patients can have received more than one prior line of systemic therapy
- ECOG performance status 0-1
- Expected survival of ≥12 weeks
Exclusion:
- No progressing CNS disease and not receiving any concurrent systemic therapy at the time of screening
- Patients with a diagnosis of a second malignancy (except prostate or cervical cancer in remission, patients with a diagnosis of basal cell carcinoma or non-muscle invasive bladder cancer, who can all be included)
- New York Heart Association Class II or greater congestive heart failure
- Patients requiring long term oxygen therapy
- Any other significant disease or disorder which, in the opinion of the investigator, may either put the participants at risk because of participation in the trial, or may influence the result of the trial, or the participant’s ability to participate in the trial
- Patients on active treatment in another clinical trial
Additional eligibility will be defined for each CST.
Funder
Each CST will be funded separately, currently CST1 is funded by BI.
Contact Information for trial queries
Senior Trial Managers: Anna Song/Katy McLaughlin
Email: selectmeso@soton.ac.uk
Phone: 023 81205154
SAE Reporting:
Email: ctu@soton.ac.uk
Phone: 0844 7740621