‘Dynamic duo’ defences in bacteria ward off viral threats
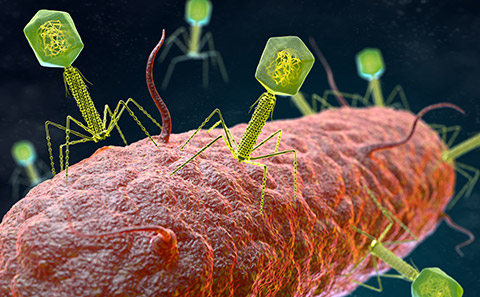
Scientists at the University of Southampton have discovered that bacteria can pair up their defence systems to create a formidable force, greater than the sum of its parts, to fight off attack from phage viruses. Understanding how bacteria react to this type of virus is a big step in combatting antimicrobial resistance.
This new groundbreaking research shows that inside each bacterial cell different defence systems are forming partnerships and combining their strengths to effectively combat viral threats. Findings of the study are published in the journal
Cell Host & Microbe
.
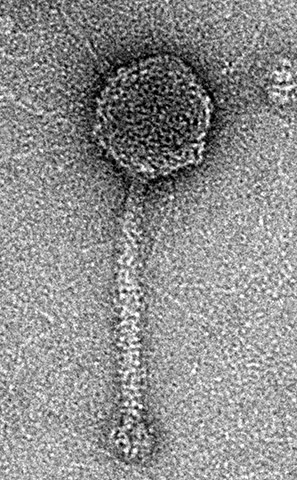
Phage viruses, or bacteriophages, could be thought of as ‘the good guys’ of the virus world. Spider-like in their appearance, the microscopic organisms can kill harmful bacteria without affecting the good bacteria in our bodies. Understanding how bacteria respond to phages is crucial in exploring how these viruses can be used to fight infections in humans, as an alternative to antibiotics.
Lead author of the study,
Dr Franklin Nobrega
of the University of Southampton, comments: “Just like how our immune system protects us from harmful germs, bacteria have their own set of defence systems which create a dynamic shield against viral threats. Imagine if your white blood cells, antibodies, and killer T-cells all joined forces to fight off a virus together. This is exactly what is happening inside bacterial cells.
“We used to think of bacterial defence as a solo act, but it turns out it’s more like a buddy system. A ‘dynamic duo’ of defence systems merge their powers to mount a stronger response than they otherwise would have achieved, potentially saving the cell from destruction.”
The researchers analysed existing datasets to find patterns of paired defence systems in the genomes (cell DNA instructions) of some 42,000 bacteria, including E. coli. They looked for pairs which occurred more often than would be expected by random chance. The scientists then took a selection of these and tested them in the lab for enhanced virus immunity and, crucially, ‘synergy’ – in other words, a defence effect in the bacteria which is more powerful than the sum of its parts.
On identifying these enhanced systems, and with further testing, they were able to see for the first time how the partnerships between individual bacterial defences are based on one system using a function from another to improve its activity. Combined, they have a more robust effect than working apart.
Antimicrobial resistance (AMR) has been identified by the World Health Organisation as one of the top ten global public health threats. It occurs when medicines, such as antibiotics, no longer effectively prevent and treat disease. Although resistance to treatments can occur naturally, the over use of certain drugs and poor infection control are accelerating the problem.
Phages could be one way of helping with AMR. Their ability to selectively kill harmful bacteria, while sparing ‘good’ bacteria, makes them a strong contender as one alternative to antibiotics. However, a lot more research is needed before treatments are refined and they can be widely used.
Dr Franklin Nobrega of the University of Southampton's
School of Biological Sciences
explains: “Phages are already in use as a last-resort treatment for antibiotic-resistant bacterial infections, a practice known as phage therapy. But by delving into how bacteria defend against these phages, we can supercharge our strategies to make them even more effective at wiping out bacterial cells, offering a glimmer of hope in the battle to keep infections at bay.”
The scientists say their research will complement efforts already underway to develop phage therapy through public participation initiatives, such as
The Phage Collection Project
and open science initiatives like
KlebPhaCol
.
The funding for this study was from the Wessex Medical Trust and the National Institutes of Health, USA.