How marine bacteria manage limited ocean iron to produce half of the oxygen on earth
The sea is the largest ecosystem on earth, and it harbours two photosynthetic organisms that produce approximately half of the oxygen on earth, fixing 45 giga-tons of carbon each year. Photosynthetic bacteria are the main contributors to global photosynthesis.
The cyanobacterium Prochlorococcus holds several records in the field: with half a micron diameter –that is 0.000,000,5 m – it is the smallest photosynthetic organism of earth, but it produces four gigatons of fixed carbon per annum, which equals to the annual net primary production of the world’s agriculture industry. However, photosynthesis depends on iron, which the bacteria must take up, and this is limiting in the ocean.
Research at Southampton University led by Associate Professor in Structural Biology Dr Ivo Tews has studied how bacteria acquire iron, by using a protein specialised in iron binding. Their surprising finding showed that two different forms or oxidation states of iron can be bound by a single protein, FutA. This reflects two essential functions, namely the uptake of ferric iron from the environment, and the protection of the bacterial photosystems as ferrous iron. As cyanobacteria typically have two types of proteins for these functions, it is speculated that the presence of a single protein in the cyanobacterium Prochlorococcus that can perform both tasks is an important factor in its ecological success.
The I24 beamline team at Diamond Light Source helped design two X-ray experiments to expose the iron binding protein to specific X-ray doses. Performed at the Diamond Light Source and the X-ray Free Electron Laser (XFEL) SACLA in Japan, the team exposed the iron binding protein to X-rays and converted rust-red iron to colourless iron. The experiments used a technique called serial crystallography, which sequentially exposes thousands of crystals briefly to the X-ray beam. These many single crystal measurements are then merged to form a complete, high-quality dataset.
Jack Stubs, Southampton PhD student at the UK XFEL hub, says: “Tickling proteins with X-rays is great fun. From the experiment we learned how changes in the protein relate to the function of binding iron in two different states.”
Robin Owen, Principal Beamline Scientist at I24 Diamond Light Source, led the development of hardware. Robin Owen said, “Close collaboration between SACLA and Diamond to exploit the complementary nature of experiments at synchrotrons and free electron lasers was key to enabling these insights.”
To locate hydrogen atoms in the structure and understand the charged state of the iron, a neutron diffraction experiment was performed at the German neutron source FRM II (Technical University of Munich). Rachel Bolton, PhD student from Southampton and the Diamond Light Source, says: “Using a nuclear reactor to see hydrogens was truly exciting to me. Even the hydrogen atoms on the water were clearly visible”.
Ivo Tews added: “This research was quite an undertaking. Three generations of PhD students and the large number of technical experts in their field that are all authors of the work speak volumes of the complexity of interdisciplinary research to decipher the fundamentals of life.”
A redox switch allows binding of Fe(II) and Fe(III) ions in the cyanobacterial iron-binding protein FutA from Prochlorococcus is published in PNAS and is available online.
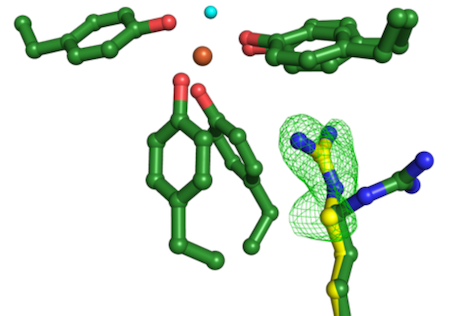
Image: The experiment performed at an XFEL X-ray Free Electron Laser in Japan (SACLA) revealed a change in the protein structure, indicated by the green mesh and the alternative position of one amino acid shown in yellow, required for the dual function of the iron binding protein.