These new insights by experts from Southampton’s Institute for Life Sciences, can be used to develop microbiome-targeted interventions to reduce adverse drug reactions and improve drug efficacy.
Exploring the gut
The human gastrointestinal tract is inhabited by trillions of microbes, single-celled organisms that play a vital role in health and wellbeing by shaping and modulating our immune systems. There can be up to 1,000 different species of microbe in each person’s gut.
Many pharmaceutical drugs can affect these microbes resulting in a number of issues, including reducing the amount of drug absorbed into the intestine, decreasing or increasing the effectiveness of the drug, and causing drug side effects.
Studies have shown that gut microbes can actively metabolise and alter the concentration of some drugs, such as widely used antidepressants and antipsychotics. While a significant number of drugs have also been shown to inhibit the growth of gut microbes.
Dr Fatima Pereira, from Biological Sciences, together with Dr Sam Thompson and Professor Sumeet Mahajan, from Chemistry, have developed a new approach to monitor these drug-microbiome interactions non-invasively and in situ.
Fatima said: “Each individual has a unique microbiome that may interact differently with a particular drug. If we are to meet the need for innovative and personalised therapeutic strategies, it is essential that we understand the proportion and identity of gut microbes that are targeted by, and/or capable of modifying drugs under physiological conditions."
To do that we need to identify which of the 1,000 different types of microbes are being affected by the drug.
Dr Fatima Pereira
Demonstrating a new approach
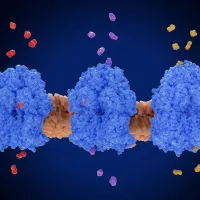
To identify the relevant microbes, the team uses an innovative combination of click-chemistry, spectroscopy and cell sorting.
Firstly, the drug is synthesised to create a ‘molecular spy’ to help detect which microbes are directly interacting with the drug. The microbes are then examined using spectroscopy to ensure the drug is interacting and working. Then a dye is added (click-chemistry) that lights up the drug inside the cells.
Fatima said: “This allows us to separate the microbes that are interacting with the drug from the microbes that are not. We then have a much smaller and more accessible group from what was originally a complex system. We use this smaller group in further experiments to identify the interactions and how drugs are actually working.”
This rapid high-throughput approach can be used to screen many healthy individuals and patients for microbiome-drug interactions.
The impact of this new research could extend to a range of therapeutic drugs, including antidepressants and drugs for cardiovascular disease.
Developing personalised medicine
Investigating the interactions of these drugs with the microbiome in a personalised way is critical, as microbiome composition and function vary greatly from person to person and contribute to the different response each individual has to drug treatments.
Understanding these interactions will improve patient health and wellbeing by opening up strategies to fine tune existing clinical drug treatments.
Fatima added: “Assessing the effects of pharmaceuticals on microbiome activity will soon be in pre-clinical drug development studies and our work will be at the forefront of such innovative approaches."
“The identification of key microbes interacting with pharmaceuticals will enable the development of precision microbiome-based interventions to improve drug efficacy and reduce adverse effects.”