About the material innovation laboratory
Material research projects, thin film synthesis and thin film characterisation
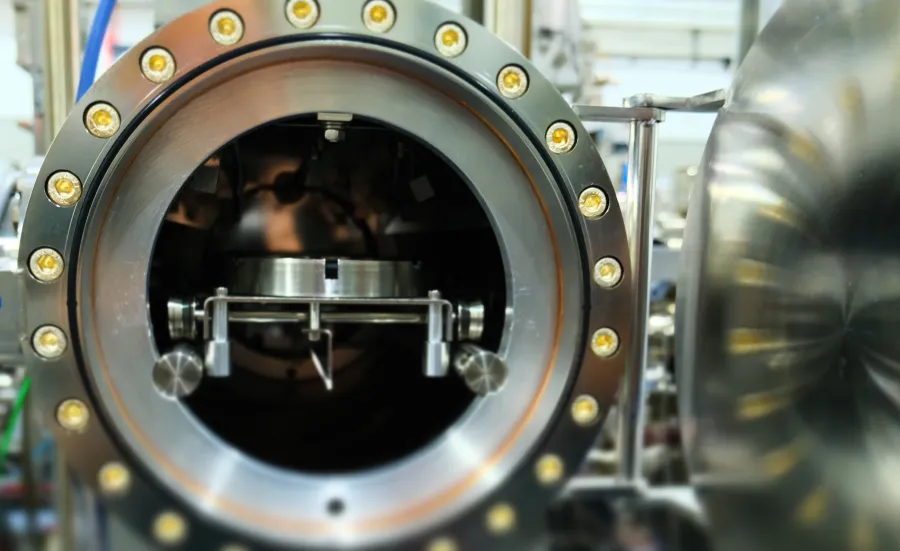
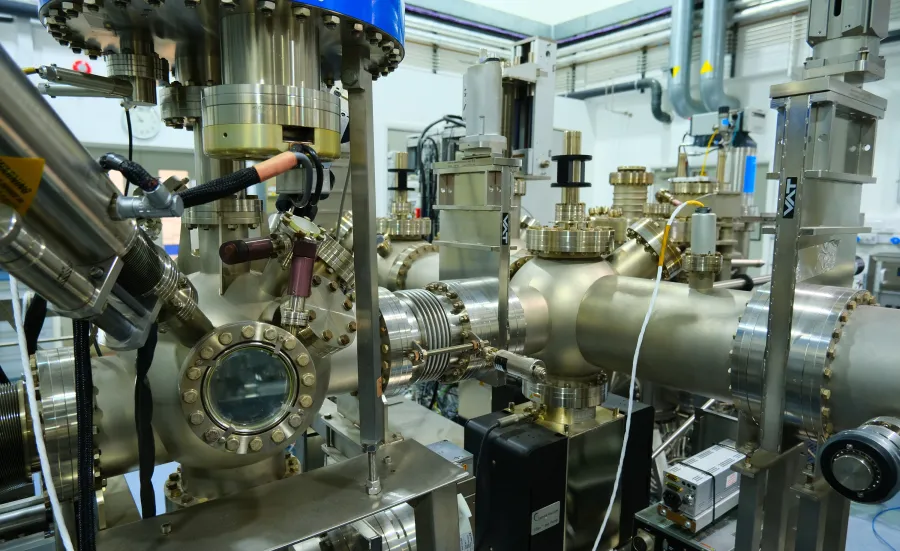
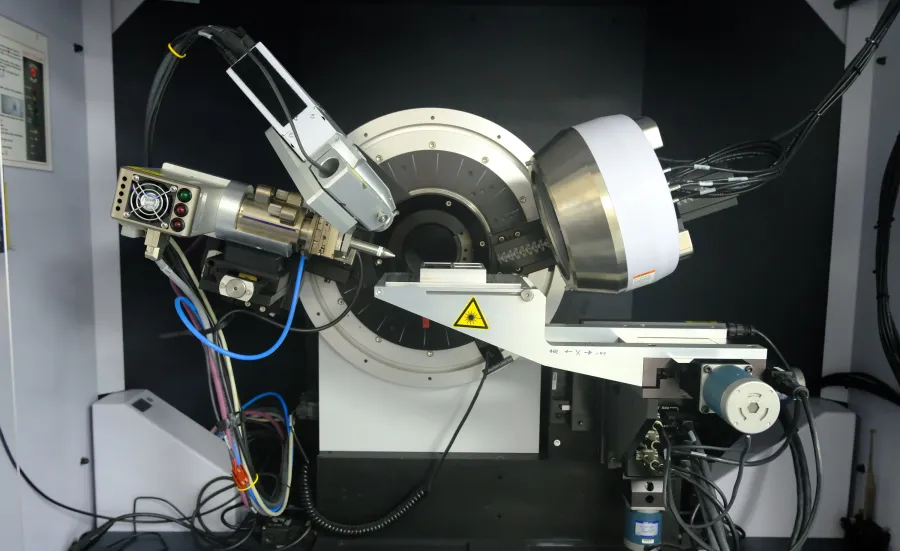
The material innovation laboratory facility offers a range of capabilities ranging from synthesis and characterisation to full research projects focusing on high-throughput methodology.
The technology and setup available at the facility allow us to rapidly explore the compositional dependence, for a given family of materials, of chosen and specific fundamental characteristics.
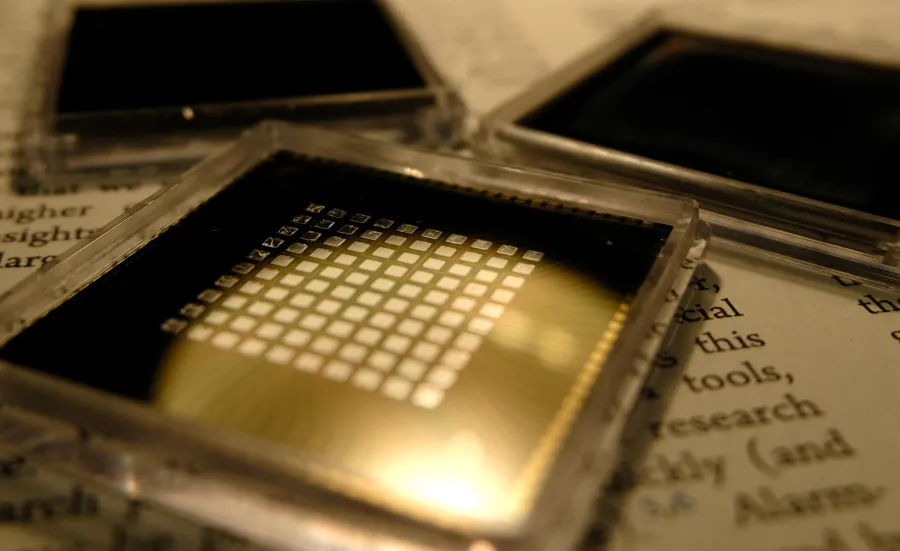
High-throughput synthesis of thin film samples is combined with automated characterisation to efficiently acquire a unique and complete dataset of the material family. Using tailored software, the data is combined and analysed to produce an understanding of the relationship between composition, crystal structure and selected physical properties.
This approach has been implemented to study phase change memory materials, advanced alloys, functional ceramics, dielectrics, metals, electrocatalysts, battery materials (anodes, cathodes and electrolytes), hydrogen storage materials, gas sensors, thermoelectrics and photovoltaics as well as optical coatings and plasmonic materials.
The facility operates as an Engineering and Physical Sciences Research Council (EPSRC) small research facility and can be included on grant applications.
It also offers a contract service for external users upon discussion with the facility manager via the nC2 Engineering Consultancy.