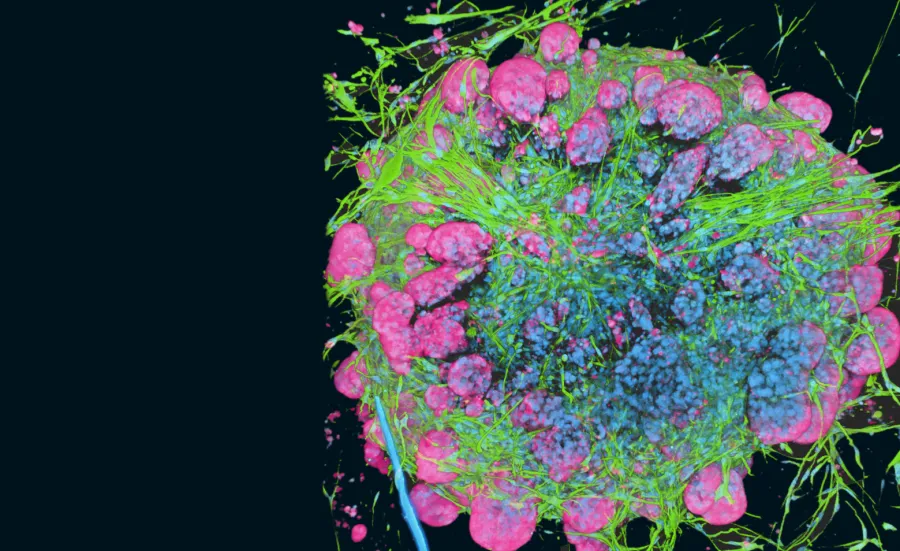
Immunotherapeutic antibodies and vaccines
We've completed numerous phase I and II trials in lymphoma, multiple myeloma and solid cancers from science developed in our labs
Lymphoid cancers
We have identified how corrupted B‑cell receptors drive lymphoma and leukaemia, revealing new targets for treatment
Antibody discovery programme
Working with Cancer Research UK and industry, we have established a pipeline of immunostimulatory reagents
Head and neck and lung cancers
We're studying myofibroblast-rich stroma to improve immunotherapy and vaccination
About Cancer Sciences at Southampton
Cancer Sciences is a school within the Faculty of Medicine. We conduct discovery, translational and clinical science and carry out clinical trials in many aspects of cancer. We are also training the next generation of cancer researchers and provide a number of research degrees including integrated PhDs.
Our strengths include:
- cancer immunology
- tumour microenvironment
- solid tumours
- lymphoid biology
- academic surgery
- clinical informatics
Our work led to the creation of the Centre for Cancer Immunology, the first of its kind in the UK. It has also led to the Cancer Immunology Fund, which helps develop early-career researchers and provide new research directions in cancer Immunology. Biotech and pharma partnerships strengthen all our activities.
We target lymphoid malignancies, gastrointestinal, paediatric/neuroblastoma, lung, head and neck, and breast cancers. Our clinical academics and trialists work closely with the:
- Clinical Informatics Research Unit
- Experimental Cancer Medicine Centre
- Southampton Clinical Trials Unit (SCTU)
- Wessex Investigational Sciences Hub laboratory
Biotherapeutics from our laboratory programs have advanced to clinical studies, in collaboration with biotech companies and the SCTU. We host a Blood Cancer UK Centre of Excellence and a Cure Leukaemia Trials Accelerator Centre for Lymphoid Malignancies.
We’re based on the same site as University Hospital Southampton which means our researchers can work closely with our clinical colleagues and access clinical material. We have strong working relationships with the NIHR Biomedical Research Centre and Wessex Cancer Alliance. Investment in surgical oncology and a tissue bank with thousands of samples support our research.
Anti-CD20 treatment
We have investigated the effector mechanism of the Food and Drug Administration-approved anti-CD20 therapeutic mAbs rituximab, ofatumumab and obinutuzumab. We showed that FcγRIIb expression on tumour targets is a strong negative prognostic factor for anti-CD20 treatment.
Explore our work improving cancer treatments through monoclonal antibodies.
Lymphoid malignancies
Our activity in this area builds on our observation of the clinical importance of the mutational status of the immunoglobulin heavy variable chains. It allows the stratification of patients with chronic lymphocytic leukaemia (CLL) into groups with discrete outcomes and treatment options. Subsequent research has enabled detailed analysis of B-cell receptor signalling in CLL and other lymphomas. We have identified somatic alterations driving CLL. We have also found novel methods for inducing selective apoptosis in CLL cells.
Breast cancer
We have discovered a new C-terminal-binding protein (CtBP) regulatory mechanism linked to metabolic status in breast cancer and the chemical synthesis of a novel class of inhibitors that target CtBP dimerization.
Oral cancer
In one of the largest UK clinical studies of its kind, we found that the strongest independent risk factor for early patient death in oral cancer patients was a myofibroblast-rich stroma. This allows early identification of aggressive cancers. We can also identify the specific mechanism cancer cells use to generate a stromal response capable of surpressing adaptive immunity.
Cervical cancer, ankylosing spondylitis and psoriasis
We were first to demonstrate functional polymorphism in the antigen processing enzyme ERAP1. This provided a mechanistic rationale for its association with diseases such as ankylosing spondylitis, psoriasis and cervical cancer.
Learn more about our treatment for gastrointestinal cancers and how we're tackling hard-to-treat cancers.
Collaboration and enterprise
We have taken a number of reagents (DNA vaccines, monoclonal antibodies, and radioimmunoconjugates) from our own laboratories into clinical trials alongside the various Pharma, biotech, research council and charities that fund our work.
We were one of the first to identify immunostimulatory mAb. We have delivered a first-in-man anti-CD40 mAb, Lob7-4, which was safe and led the way for subsequent research in this area.
With Cancer Research UK, we established an antibody discovery programme. This generates a pipeline of immunostimulatory reagents, which target the immune co-receptors of the TNFR superfamily.
The first, an anti-CD27, has been developed in partnership with Celldex therapeutics, and is now in phase I trials. Our work on the mechanism of anti-CD20 and FcgRIIb antibodies led to a new collaborative initiative with the Swedish Biotech company: BioInvent International.
We were the first to define the immunological functions for the MHC I cofactors calreticulin and tapasin. We were also first to relate these to the generation of immunodominance to experimental vaccines.
In collaboration with Microsoft, we developed a new computational model that helps predict the immunological outcome of various immunotherapeutic approaches.
Research themes
Our research themes allow us to:
- develop novel antibody therapeutics to elicit anti-cancer immunity
- modify tumour recognition by the immune system
- study the tumour microenvironment's role in progression and immune evasion
- investigate lymphoid cancer progression and therapeutic exploitation
- integrate healthcare data with molecular profiling
They cover 3 main areas of investigation in cancer immunology, biology and clinical practice.
Cancer immunology
Cancer biology
Cancer clinical and surgery
- Clinical and medical oncology
- Experimental Cancer Medicine Centre (ECMC)
- Haematology
- Lymphoma
- Southampton Clinical Trials Unit
- Surgery
Contribute to the field of cancer sciences by completing a postgraduate research degree:
Our research
The School of Cancer Sciences hosts a number of research centres. Get to know our community and learn about the impact of our work.
-

Medicine Research
We make important scientific discoveries in the lab and translate them into successful clinical treatments.
-

Centre of Cancer Immunology
Explore the full detail of our projects, publications and the impact of our research.
-

Southampton Clinical Trials Unit
We deliver world-leading clinical trials of innovative new treatments and diagnostic tools. Our trials directly influence routine clinical practice for the benefit of patients.
-
Antibody and Vaccine Group
We are a collaborative group of about 60 scientists and clinician scientists working to a common goal: how to harness the immune system to cure cancer.
-

Bioinformatics and Genomics
We explore new technologies and treatments to promote the repair and regeneration of bones and cartilage.
-
Cancer B-Cell Group
Our research translates new understanding of tumour B-cell biology into clinical practice.
-
Clinical Cancer Researchers
We are dedicated to advancing cancer diagnosis and treatment.
-

Clinical Informatics Research Unit
We deliver healthcare innovation through informatics solutions, using applied research in software development, data modelling and definitions.
-

Experimental Cancer Medicine
We deliver access to world class experimental cancer medicine for the population of southern England and beyond.